ProLung™-budesonide Inhibits SARS-CoV-2 Replication and Reduces Lung Inflammation
et al., bioRxiv, doi:10.1101/2021.05.05.442779, May 2021
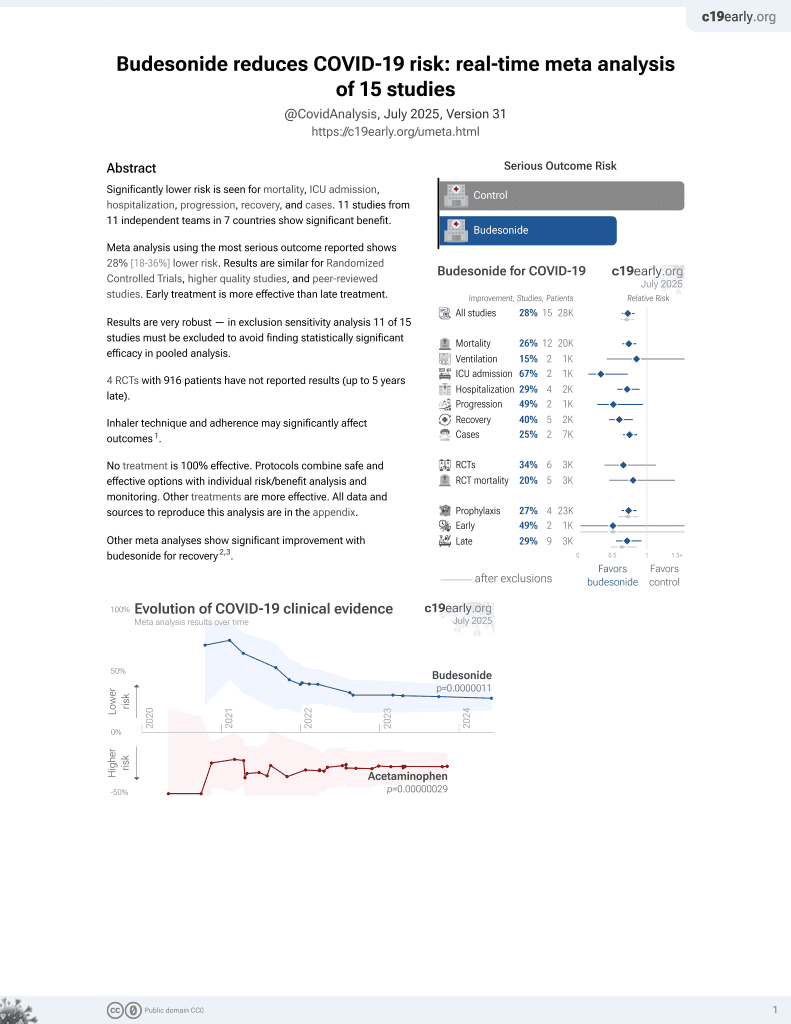
Budesonide for COVID-19
27th treatment shown to reduce risk in
September 2021, now with p = 0.0000042 from 14 studies, recognized in 10 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study and animal study showing that ProLung™-budesonide inhibits SARS-CoV-2 replication (results for budesonide were not provided). ProLung™-budesonide and budesonide significantly decreased lung inflammation. ProLung™-budesonide is a formulation for sustained administration of a low dose of budesonide using a vehicle similar to lung surfactant.
3 preclinical studies support the efficacy of budesonide for COVID-19:
Konduri et al., 5 May 2021, preprint, 10 authors.
ProLung™-budesonide Inhibits SARS-CoV-2 Replication and Reduces Lung Inflammation
doi:10.1101/2021.05.05.442779
Background: Inhaled budesonide benefits patients with COVID-19. ProLung™-budesonide enables the sustained, low dose administration of budesonide within a delivery vehicle similar to lung surfactant. ProLung™-budesonide may offer anti-inflammatory and protective effects to the lung in COVID-19, yet it's effect on SARS-CoV-2 replication is unknown. Objective: To determine the efficacy of ProLung™-budesonide against SARS-CoV-2 infection in vitro, evaluate its ability to decrease inflammation, and airway hyperresponsiveness in an animal model of lung inflammation. Methods: SARS-CoV-2-infected Vero 76 cells were treated with ProLung™-budesonide ([0.03-100 µg/ml]) for 3 days, and virus yield in the supernatant was measured. Ovalbumin-sensitized C57BL/6 mice received aerosolized (a) ProLung™-budesonide weekly, (b) only budesonide, either daily or weekly, or (c) weekly empty ProLung™-carrier (without budesonide). All treatment groups were compared to sensitized untreated, or normal mice using histopathologic examination,
TABLES AND FIGURES
References
Bhattacharya, Westphalen, Macrophage-epithelial interactions in pulmonary alveoli, Semin Immunopathol, doi:10.1007/s00281-016-0569-x
Chung, Beiss, Fiering, Steinmetz, COVID-19 Vaccine Frontrunners and Their Nanotechnology Design, ACS Nano, doi:10.1021/acsnano.0c07197
Düzgüneş, Pretzer, Simões, Slepushkin, Konopka et al., Liposome-mediated delivery of antiviral agents to human immunodeficiency virus-infected cells, Mol Membr Biol, doi:10.1080/096876899294832
Gabizon, Nanoparticle Interactions with the Immune System: Clinical Implications for Liposome-Based Cancer Chemotherapy, Front Immunol, doi:10.3389/fimmu.2017.00416
Gangadharam, Ashtekar, Flasher, Düzgüneş, Therapy of Mycobacterium avium complex infections in beige mice with streptomycin encapsulated in sterically was not certified by peer review) is the author/funder, Antimicrob Agents Chemother
George, Barratt, Condliffe, Desai, Devaraj et al., Respiratory follow-up of patients with COVID-19 pneumonia, Thorax
Grifoni, Valoriani, Cei, Lamanna, Gelli et al., Interleukin-6 as prognosticator in patients with COVID-19, J Infect, doi:10.1016/j.jinf.2020.06.008
Hafner, Corthésy, Merkle, Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant, Adv Drug Deliv Rev, doi:10.1016/j.addr.2013.05.013
He, Yao, Chen, Wang, Fang et al., The poor prognosis and influencing factors of high D-dimer levels for COVID-19 patients, Sci Rep, doi:10.1038/s41598-021-81300-w
Jung, Nam, Park, Lee, Hong et al., Protective effect of phosphatidylcholine on lipopolysaccharide-induced acute inflammation in multiple organ injury, Korean J Physiol Pharmacol, doi:10.4196/kjpp.2013.17.3.209
Konduri, Nandedkar, Düzgünes, Suzara, Artwohl et al., Efficacy of liposomal budesonide in experimental asthma, J Allergy Clin Immunol, doi:10.1067/mai.2003.104
Konduri, Nandedkar, Rickaby, Düzgüneş, Gangadharam, The use of sterically stabilized liposomes to treat asthma, Methods Enzymol, doi:10.1016/S0076-6879(05)91023-9
Majumdar, Flasher, Friend, Nassos, Yajko et al., Efficacies of liposome-encapsulated streptomycin and ciprofloxacin against Mycobacterium avium-M. intracellulare complex infections in human peripheral blood monocyte/macrophages, Antimicrob Agents Chemother, doi:10.1128/aac.36.12.2808
Mason, Dobbs, Synthesis of phosphatidylcholine and phosphatidylglycerol by alveolar type II cells in primary culture, J Biol Chem
Monteil, Kwon, Prado, Hagelkrüys, Wimmer et al., Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2, Cell, doi:10.1016/j.cell.2020.04.004
Morris, Bortolasci, Puri, Olive, Marx et al., The pathophysiology of SARS-CoV-2: A suggested model and therapeutic approach, Life Sci, doi:10.1016/j.lfs.2020.118166
Nguyen, Rajaram, Meyer, Schlesinger, Pulmonary surfactant protein A and surfactant lipids upregulate IRAK-M, a negative regulator of TLR-mediated inflammation in human macrophages, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00067.2012
Reed, Muench, A Simple Method of Estimating Fifty Percent Endpoints, Am J Hyg
Schousboe, Wiese, Heiring, Verder, Poorisrisak et al., Assessment of pulmonary surfactant in COVID-19 patients, Crit Care, doi:10.1186/s13054-020-03268-9
Song, Tang, Yin, Combination antitumor immunotherapy with VEGF and PIGF siRNA via systemic delivery of multi-functionalized nanoparticles to tumor-associated macrophages and breast cancer cells, Biomaterials, doi:10.1016/j.biomaterials.2018.09.017
Wang, Xie, Zhao, Fei, Zhang et al., Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients, EBioMedicine, doi:10.1016/j.ebiom.2020.102833
Wiedermann, Lederer, Mayr, Sepp, Herold et al., Prospective observational study of antiphospholipid antibodies in acute lung injury and acute respiratory distress syndrome: comparison with catastrophic antiphospholipid syndrome, Lupus, doi:10.1191/0961203303lu413oa
DOI record:
{
"DOI": "10.1101/2021.05.05.442779",
"URL": "http://dx.doi.org/10.1101/2021.05.05.442779",
"abstract": "<jats:title>ABSTRACT</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Inhaled budesonide benefits patients with COVID-19. <jats:italic>ProLung™-budesonide</jats:italic> enables the sustained, low dose administration of budesonide within a delivery vehicle similar to lung surfactant. <jats:italic>ProLung™-budesonide</jats:italic> may offer anti-inflammatory and protective effects to the lung in COVID-19, yet it’s effect on SARS-CoV-2 replication is unknown.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To determine the efficacy of <jats:italic>ProLung™-budesonide</jats:italic> against SARS-CoV-2 infection in vitro, evaluate its ability to decrease inflammation, and airway hyperresponsiveness in an animal model of lung inflammation.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>SARS-CoV-2-infected Vero 76 cells were treated with <jats:italic>ProLung™-budesonide</jats:italic> ([0.03– 100 μg/ml]) for 3 days, and virus yield in the supernatant was measured. Ovalbumin-sensitized C57BL/6 mice received aerosolized (a) <jats:italic>ProLung™-budesonide</jats:italic> weekly, (b) only budesonide, either daily or weekly, or (c) weekly empty <jats:italic>ProLung™-carrier</jats:italic> (without budesonide). All treatment groups were compared to sensitized untreated, or normal mice using histopathologic examination, electron microscopy (EM), airway hyperresponsiveness (AHR) to Methacholine (Mch) challenge, and eosinophil peroxidase activity (EPO) measurements in bronchioalveolar lavage (BAL).</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p><jats:italic>ProLung™-budesonide</jats:italic> showed significant inhibition on viral replication of SARS-CoV-2-infected cells with the selectivity index (SI) value > 24. Weekly <jats:italic>ProLung™-budesonide</jats:italic> and daily budesonide therapy significantly decreased lung inflammation and EPO in BAL. <jats:italic>ProLung™-budesonide</jats:italic> localized in type II pneumocytes, and was the only group to significantly decrease AHR, and EPO in BAL with Mch challenge</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p><jats:italic>ProLung™-budesonide</jats:italic> significantly inhibited viral replication in SARS-CoV-2 infected cells. It localized into type II pneumocytes, decreased lung inflammation, AHR and EPO activity with Mch challenge. This novel drug formulation may offer a potential inhalational treatment for COVID-19.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2021,
5,
5
]
]
},
"author": [
{
"affiliation": [],
"family": "Konduri",
"given": "Kameswari S.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Pattisapu",
"given": "Ram",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pattisapu",
"given": "Jogi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Konduri",
"given": "Girija G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zwetchkenbaum",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roy",
"given": "Bidhan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barman",
"given": "Monalisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frazier",
"given": "Adria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hurst",
"given": "Brett L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Düzgüneş",
"given": "Nejat",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
5,
6
]
],
"date-time": "2021-05-06T04:50:20Z",
"timestamp": 1620276620000
},
"deposited": {
"date-parts": [
[
2021,
5,
7
]
],
"date-time": "2021-05-07T20:28:56Z",
"timestamp": 1620419336000
},
"group-title": "Pharmacology and Toxicology",
"indexed": {
"date-parts": [
[
2022,
3,
29
]
],
"date-time": "2022-03-29T00:05:36Z",
"timestamp": 1648512336198
},
"institution": [
{
"name": "bioRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2021,
5,
5
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.05.05.442779",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
5,
5
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
5,
5
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1038/s41598-021-81300-w",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.1"
},
{
"DOI": "10.1016/j.jinf.2020.06.008",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.2"
},
{
"DOI": "10.1016/j.cell.2020.04.004",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.3"
},
{
"DOI": "10.1016/j.tcb.2020.09.006",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.4"
},
{
"DOI": "10.1007/s11684-020-0754-0",
"article-title": "Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection",
"doi-asserted-by": "crossref",
"first-page": "185",
"issue": "2",
"journal-title": "Front Med.",
"key": "2021050713250763000_2021.05.05.442779v1.5",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1111/ijcp.13943",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.6"
},
{
"DOI": "10.1016/S2213-2600(21)00160-0",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.7"
},
{
"DOI": "10.1016/j.jaci.2020.09.034",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.8"
},
{
"DOI": "10.1067/mai.2003.104",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.9"
},
{
"DOI": "10.1191/0961203303lu413oa",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.10"
},
{
"DOI": "10.1186/s13054-020-03268-9",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.11"
},
{
"DOI": "10.1152/ajplung.00067.2012",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.12"
},
{
"DOI": "10.4196/kjpp.2013.17.3.209",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.13"
},
{
"DOI": "10.1002/prot.22879",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.14"
},
{
"DOI": "10.1128/AAC.39.3.725",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.15"
},
{
"DOI": "10.1016/S0076-6879(05)91023-9",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.16"
},
{
"DOI": "10.1016/S0021-9258(19)70755-8",
"article-title": "Synthesis of phosphatidylcholine and phosphatidylglycerol by alveolar type II cells in primary culture",
"doi-asserted-by": "crossref",
"first-page": "5101",
"issue": "11",
"journal-title": "J Biol Chem.",
"key": "2021050713250763000_2021.05.05.442779v1.17",
"volume": "255",
"year": "1980"
},
{
"DOI": "10.1016/j.ebiom.2020.102833",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.18"
},
{
"DOI": "10.1016/j.lfs.2020.118166",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.19"
},
{
"DOI": "10.1007/s00281-016-0569-x",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.20"
},
{
"DOI": "10.1080/096876899294832",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.21"
},
{
"DOI": "10.1128/AAC.36.12.2808",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.22"
},
{
"DOI": "10.1016/j.addr.2013.05.013",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.23"
},
{
"DOI": "10.1016/j.biomaterials.2018.09.017",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.24"
},
{
"DOI": "10.1021/acsnano.0c07197",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.25"
},
{
"DOI": "10.3389/fimmu.2017.00416",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.26"
},
{
"DOI": "10.1136/thoraxjnl-2020-215314",
"doi-asserted-by": "publisher",
"key": "2021050713250763000_2021.05.05.442779v1.27"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "http://biorxiv.org/lookup/doi/10.1101/2021.05.05.442779"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "<i>ProLung</i>™-<i>budesonide</i> Inhibits SARS-CoV-2 Replication and Reduces Lung Inflammation",
"type": "posted-content"
}