Effects of Azvudine on the Low-Risk Patients Infected with COVID-19 Omicron Variants: A Retrospective Case-Control Study
et al., Journal of Clinical Pharmacology and Therapeutics, 5:1, Feb 2024
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
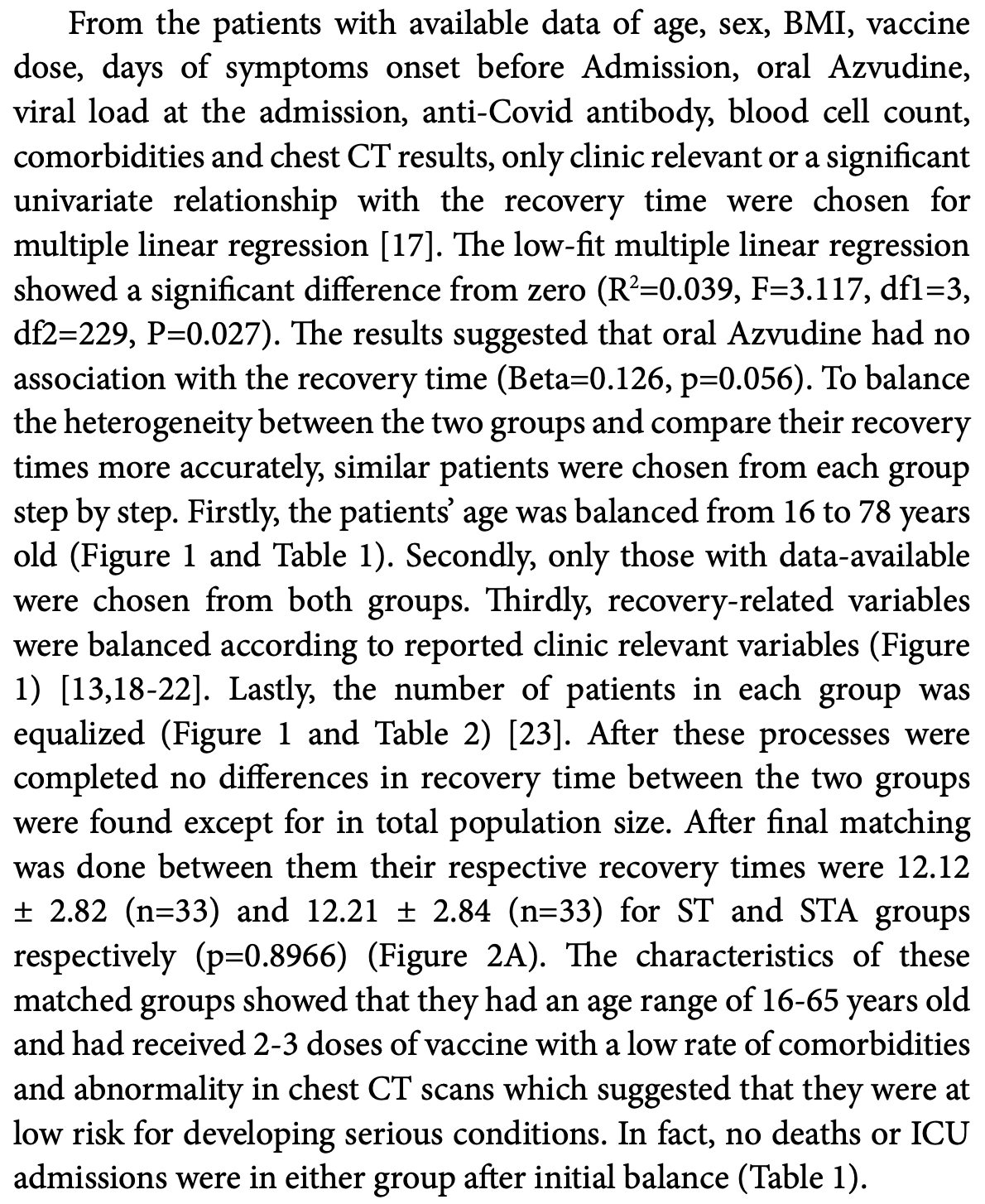
Retrospective 481 low-risk COVID-19 patients in China showing no significant difference in recovery or symptomatic severity with azvudine, but slightly lower total viral load.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
recovery time, 0.7% higher, relative time 1.01, p = 0.90, treatment mean 12.21 (±2.84) n=33, control mean 12.12 (±2.82) n=33.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Jin et al., 12 Feb 2024, retrospective, China, peer-reviewed, 14 authors.
Effects of Azvudine on the Low-Risk Patients Infected with COVID-19 Omicron Variants: A Retrospective Case-Control Study Research Article
Objective: To evaluate the efficacy and safety of Azvudine in treatment of the patients infected with COVID-19 Omicron variants.
Methods: This study included the discharged patients after COVID-19 infection from October 17 to November 17 in 2022 in Zhengzhou Central Hospital. The patients were divided into two groups, the Symptomatic Treatment group (ST) and the Symptomatic Treatment and oral Azvudine (STA) groups to evaluate the efficacy and safety of Azvudine. Results: A total 481 patients were included. The recovery time had no correlation with oral Azvudine (Beta=1.920, p=0.056) in a low-fit multiple linear regression with the data-available patients (R2=0.039, F=3.117, p=0.027). No significant differences were found in the recovery time (12.12 ± 2.83 vs. 12.21 ± 2.84, n=33, P=0.897) and symptomatic severity between the two groups after 1:1 matched. However, STA groups had lower total viral load than ST group after the final matching (28.03 ± 4.72 vs. 25.53 ± 5.32, n=33, P=0.048). Seventeen of 206 patients reported Azvudine-related adverse effects and stopped Azvudine.
Conclusion: Azvudine had little effect on the low-risk patients with Omicron infection to improve recovery time and symptoms. However, it could slightly decrease total viral load during the first 5 days after administration while being relatively safe for oral use overall.
References
Cai, Deng, Yang, Sun, Liu et al., Modeling transmission of SARS-CoV-2 Omicron in China, Nat Med
Chu, Sun, Bai, Bai, Zhang et al., Combined spinal-epidural analgesia and epidural analgesia induced maternal fever with a similar timing during labor-A randomized controlled clinical trial, Front Med
Dadras, Afsahi, Pashaei, Mojdeganlou, Karimi et al., The relationship between COVID-19 viral load and disease severity: A systematic review, Immun Inflamm Dis
Drozdzal, Rosik, Lechowicz, Machaj, Szostak et al., An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment, Drug Resist Updat
Fayzullina, Kharwar, Acharya, Buzdin, Borisov et al., FNC: An Advanced Anticancer Therapeutic or Just an Underdog?, Front Oncol
Feng, Shen, Li, Li, Wang et al., The First Outbreak of Omicron Subvariant BA.5.2 -Beijing Municipality, China, China CDC Wkly
Fernandes, Inchakalody, Merhi, Mestiri, Taib et al., Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines, Ann Med
Fiolet, Kherabi, Macdonald, Ghosn, Peiffer-Smadja, Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review, Clin Microbiol Infect
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med
Kandeel, Mohamed, El-Lateef, Venugopala, El-Beltagi, Omicron variant genome evolution and phylogenetics, J Med Virol
Karim, Karim, Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic, Lancet
Knoll, Schultze, Schulte-Schrepping, Monocytes and Macrophages in COVID-19, Front Immunol
Li, Wang, Clercq, Approved HIV reverse transcriptase inhibitors in the past decade, Acta Pharm Sin B
Long, Carius, Chavez, Liang, Brady et al., Clinical update on COVID-19 for the emergency clinician: Presentation and evaluation, Am J Emerg Med
Malden, Hong, Lewin, Ackerson, Lipsitch et al., Hospitalization and Emergency Department Encounters for COVID-19 After Paxlovid Treatment -California, December 2021-May 2022, MMWR Morb Mortal Wkly Rep
Meo, Meo, Ff, Klonoff, Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics, Eur Rev Med Pharmacol Sci
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients, Clin Infect Dis
Po, Omicron variant as nature's solution to the COVID-19 pandemic, J Clin Pharm Ther
Reis, Metzendorf, Kuehn, Popp, Gagyor et al., Nirmatrelvir combined with ritonavir for preventing and treating COVID-19, Cochrane Database Syst Rev
Stone, Maehara, Lansky, De Bruyne, Cristea et al., Investigators, A prospective natural-history study of coronary atherosclerosis, N Engl J Med
Tsang, Chan, Cho, Yu, Yim et al., An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies, Expert Rev Anti Infect Ther
Wang, Zhao, Liu, Chen, Feng, Early administration of Paxlovid reduces the viral elimination time in patients infected with SARS-CoV-2 Omicron variants, J Med Virol
Yu, Chang, The first Chinese oral anti-COVID-19 drug Azvudine launched, Innovation (Camb)
Zeng, Lv, Liu, Jiang, Huang et al., Clinical Characteristics of Omicron SARS-CoV-2 Variant Infection After Non-mRNA-Based Vaccination in China, Front Microbiol
Zhang, Li, Wang, Liu, Lu et al., Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct Target Ther
Zhang, Zhang, Chen, Shanghai's life-saving efforts against the current omicron wave of the COVID-19 pandemic, Lancet
Zhu, Efficacy and safety evaluation of Azvudine in the prospective treatment of COVID-19 based on four phase III clinical trials, Front Pharmacol
