Influence of androgen deprivation therapy on the severity of COVID-19 in prostate cancer patients
et al., The Prostate, doi:10.1002/pros.24232, Sep 2021
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
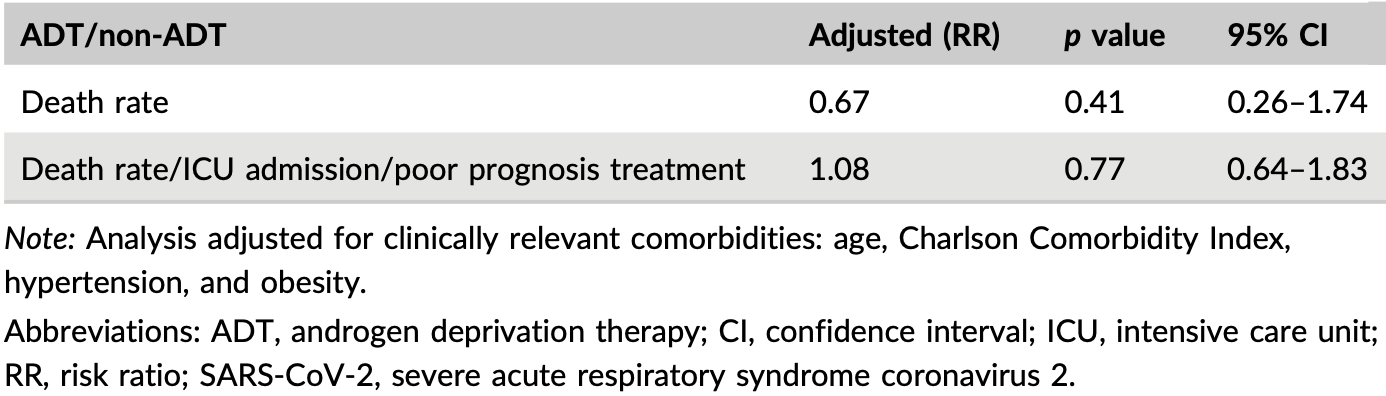
Retrospective 1,349 prostate cancer patients in Spain, 156 on ADT, showing no significant differences in COVID-19 outcomes with treatment.
Although the 33% lower mortality is not statistically significant, it is consistent with the significant 37% lower mortality [21‑50%] from meta-analysis of the 32 mortality results to date.
|
risk of death, 33.0% lower, RR 0.67, p = 0.41, treatment 3 of 11 (27.3%), control 17 of 50 (34.0%), adjusted per study, multivariable.
|
|
risk of progression, 8.0% higher, RR 1.08, p = 0.77, treatment 11, control 50, adjusted per study, multivariable.
|
|
risk of case, 68.2% higher, RR 1.68, p = 0.15, treatment 11 of 156 (7.1%), control 50 of 1,193 (4.2%), excluded in exclusion analyses:
excessive unadjusted differences between groups.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Jiménez-Alcaide et al., 13 Sep 2021, retrospective, Spain, peer-reviewed, 9 authors.
Influence of androgen deprivation therapy on the severity of COVID‐19 in prostate cancer patients
The Prostate, doi:10.1002/pros.24232
Background: The TMPRSS2 protein has been involved in severe acute respiratory syndrome caused by coronavirus 2 (SARS-CoV-2). The production is regulated by the androgen receptor (AR). It is speculated that androgen deprivation therapy (ADT) may protect patients affected by prostate cancer (PC) from SARS-CoV-2 infection. Methods: This is a retrospective study of patients treated for COVID-19 in our institution who had a previous diagnosis of PC. We analyzed the influence of exposure of ADT on the presence of severe course of COVID-19. Results: A total of 2280 patients were treated in our center for COVID-19 with a worse course of disease in males (higher rates of hospitalization, intense care unit [ICU] admission, and death). Out of 1349 subjects registered in our PC database, 156 were on ADT and 1193 were not. Out of those, 61 (4.52%) PC patients suffered from COVID-19, 11 (18.0%) belonged to the ADT group, and 50 (82.0%) to the non-ADT group. Regarding the influence of ADT on the course of the disease, statistically significant differences were found neither in the death rate (27.3% vs. 34%; p = 0.481), nor in the presence of severe COVID-19: need for intubation or ICU admission (0% vs. 6.3%; p = 0.561) and need for corticoid treatment, interferon beta, or tocilizumab (60% vs. 34.7%; p = 0.128). Multivariate analysis adjusted for clinically relevant comorbidities did not find that ADT was a protective factor for worse clinical evolution (risk ratio [RR] 1.08; 95% confidence interval [CI], 0.64-1.83; p = 0.77) or death (RR, 0.67; 95% CI, 0.26-1.74; p = 0.41). Conclusions: Our study confirms that COVID-19 is more severe in men. However, the use of ADT in patients with PC was not shown to prevent the risk of severe COVID-19.
CONFLICT OF INTERESTS The authors declare that there are no conflict of interests.
References
Arora, Schenkein, Murali, Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade, Cell
Bugge, Antalis, Wu, Type II transmembrane serine proteases, J Biol Chem
Caffo, Zagonel, Baldessari, On the relationship between androgen-deprivation therapy for prostate cancer and risk of infection by SARS-CoV-2, Ann Oncol, doi:10.1016/j.annonc.2020.06.005
Cai, Sex difference and smoking predisposition in patients with COVID-19, Lancet Respir Med, doi:10.1016/S2213-2600(20)30117-X
Choi, Bertram, Glowacka, Park, Pohlmann, Type II transmembrane serine proteases in cancer and viral infections, Trends Mol Med
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Jiménez-Alcaide, García-Fuentes, Hernández, Influence of androgen deprivation therapy on the severity of COVID-19 in prostate cancer patients, The Prostate
Klein, Flanagan, Sex differences in immune responses, Nat Rev Immunol
Klein, Li, Milinovich, Androgen deprivation therapy in men with prostate cancer does not affect risk of infection with SARS-CoV-2, J Urol, doi:10.1097/JU.0000000000001338
Lucas, Heinlein, Kim, The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis, Cancer Discov
Lucas, True, Hawley, The androgen-regulated type II serine protease TMPRSS2 is differentially expressed and mislocalized in prostate adenocarcinoma, J Pathol
Matsuyama, Nao, Shirato, Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells, Proc Natl Acad Sci
Mikkonen, Pihlajamaa, Sahu, Zhang, Janne, Androgen receptor and androgen-dependent gene expression in lung, Mol Cell Endocrinol
Montopoli, Zumerle, Vettor, Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a populationbased study (N = 4532), Ann Oncol
Patel, Zhong, Liaw, Does androgen deprivation therapy protect against severe complications from COVID-19?, Ann Oncol, doi:10.1016/j.annonc.2020.06.023
Shaw, Whitaker, Corcoran, The early effects of rapid androgen deprivation on human prostate cancer, Eur Urol
Shaw, Whitaker, Corcoran, The early effects of rapid androgen deprivation on human prostate cancer, Eur Urol
Shin, Seong, Type II transmembrane serine proteases as potential target for anti-influenza drug discovery, Expert Opin Drug Discov
Zhang, Xie, Hashimoto, Current status of potential therapeutic candidates for the COVID-19 crisis, Brain Behav Immun, doi:10.1016/j.bbi.2020.04.046
Zhou, Yang, Wang, A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature
Zhu, Zhang, Wang, A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med
Zou, A modified Poisson regression approach to prospective studies with binary data, Am J Epidemiol
DOI record:
{
"DOI": "10.1002/pros.24232",
"ISSN": [
"0270-4137",
"1097-0045"
],
"URL": "http://dx.doi.org/10.1002/pros.24232",
"alternative-id": [
"10.1002/pros.24232"
],
"archive": [
"Portico"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-03-25"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-08-30"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-09-13"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0941-1485",
"affiliation": [
{
"name": "Department of Urology Hospital Universitario Fundación Alcorcón Madrid Spain"
}
],
"authenticated-orcid": false,
"family": "Jiménez‐Alcaide",
"given": "Estíbaliz",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Urology Hospital Universitario Fundación Alcorcón Madrid Spain"
}
],
"family": "García‐Fuentes",
"given": "Clara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Urology Hospital Universitario Fundación Alcorcón Madrid Spain"
}
],
"family": "Hernández",
"given": "Virginia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Urology Hospital Universitario Fundación Alcorcón Madrid Spain"
}
],
"family": "De la Peña",
"given": "Enrique",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Research Unit Hospital Universitario Fundación Alcorcón Madrid Spain"
}
],
"family": "Pérez‐Fernández",
"given": "Elia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Urology Hospital Universitario Fundación Alcorcón Madrid Spain"
}
],
"family": "Castro",
"given": "Alejandro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Radiation Oncology Hospital Universitario de Fuenlabrada Madrid Spain"
}
],
"family": "Caballero‐Perea",
"given": "Begoña",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Urology Hospital Universitario Fundación Alcorcón Madrid Spain"
}
],
"family": "Guijarro",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Urology Hospital Universitario Fundación Alcorcón Madrid Spain"
}
],
"family": "Llorente",
"given": "Carlos",
"sequence": "additional"
}
],
"container-title": [
"The Prostate"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2021,
9,
13
]
],
"date-time": "2021-09-13T19:38:21Z",
"timestamp": 1631561901000
},
"deposited": {
"date-parts": [
[
2021,
11,
2
]
],
"date-time": "2021-11-02T08:32:35Z",
"timestamp": 1635841955000
},
"indexed": {
"date-parts": [
[
2021,
12,
10
]
],
"date-time": "2021-12-10T00:05:28Z",
"timestamp": 1639094728698
},
"is-referenced-by-count": 1,
"issn-type": [
{
"type": "print",
"value": "0270-4137"
},
{
"type": "electronic",
"value": "1097-0045"
}
],
"issue": "16",
"issued": {
"date-parts": [
[
2021,
9,
13
]
]
},
"journal-issue": {
"issue": "16",
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
13
]
],
"date-time": "2021-09-13T00:00:00Z",
"timestamp": 1631491200000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
13
]
],
"date-time": "2021-09-13T00:00:00Z",
"timestamp": 1631491200000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/pros.24232",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/pros.24232",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/pros.24232",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "1349-1354",
"prefix": "10.1002",
"published": {
"date-parts": [
[
2021,
9,
13
]
]
},
"published-online": {
"date-parts": [
[
2021,
9,
13
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_2_1"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_3_1"
},
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_4_1"
},
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_5_1"
},
{
"key": "e_1_2_8_6_1",
"unstructured": "Johns Hopkins Coronavirus Resource Center. COVID‐19 Map.https://coronavirus.jhu.edu/map.html. Accessed February 2021."
},
{
"DOI": "10.1080/17460441.2017.1372417",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_7_1"
},
{
"DOI": "10.1016/j.molmed.2009.05.003",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_8_1"
},
{
"DOI": "10.1074/jbc.R109.021006",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_9_1"
},
{
"DOI": "10.1073/pnas.2002589117",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_10_1"
},
{
"DOI": "10.1016/j.eururo.2015.10.042",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_11_1"
},
{
"DOI": "10.1158/2159-8290.CD-13-1010",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_12_1"
},
{
"DOI": "10.1002/path.2330",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_13_1"
},
{
"DOI": "10.1016/j.mce.2009.12.022",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_14_1"
},
{
"DOI": "10.1016/j.bbi.2020.04.046",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_15_1"
},
{
"DOI": "10.1093/aje/kwh090",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_16_1"
},
{
"DOI": "10.1016/j.eururo.2015.10.042",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_17_1"
},
{
"DOI": "10.1038/nri.2016.90",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_18_1"
},
{
"DOI": "10.1016/j.cell.2013.11.012",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_19_1"
},
{
"DOI": "10.1016/S2213-2600(20)30117-X",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_20_1"
},
{
"DOI": "10.1016/j.annonc.2020.04.479",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_21_1"
},
{
"DOI": "10.1097/JU.0000000000001338",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_22_1"
},
{
"DOI": "10.1016/j.annonc.2020.06.005",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_23_1"
},
{
"DOI": "10.1016/j.annonc.2020.06.023",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_24_1"
},
{
"key": "e_1_2_8_25_1",
"unstructured": "ClinicalTrials.gov. Identifier NCT 04729491 Early Antiandrogen Treatment (EAT) With Dutasteride for COVID‐19 (EAT‐DUTA AndroCoV Trial) [Internet];2000. Bethesda MD: National Library of Medicine (US). Accessed May 20 2021.https://clinicaltrials.gov/ct2/show/NCT04729491?cond=COVID19%2Bandrogen%2Bdeprivation%2Btherapy%26draw=2%26rank=2"
},
{
"key": "e_1_2_8_26_1",
"unstructured": "ClinicalTrials.gov. Identifier NCT04446429 Anti‐Androgen Treatment for COVID‐19 [Internet];2000. Bethesda (MD): National Library of Medicine (US). Accessed May 20 2021.https://clinicaltrials.gov/ct2/show/results/NCT04446429?cond=Covid-19%2BProstate%2Bcancer%2BAND%2B%22Prostatic%2BNeoplasms%22%26draw=2%26rank=1"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"score": 1,
"short-container-title": [
"The Prostate"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Urology",
"Oncology"
],
"subtitle": [],
"title": [
"Influence of androgen deprivation therapy on the severity of COVID‐19 in prostate cancer patients"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "81"
}
