Effect of Convalescent Plasma Therapy on Clinical Improvement of COVID-19 Patients: A Randomized Clinical Trial
et al., Tanaffos 21:1, Jan 2022
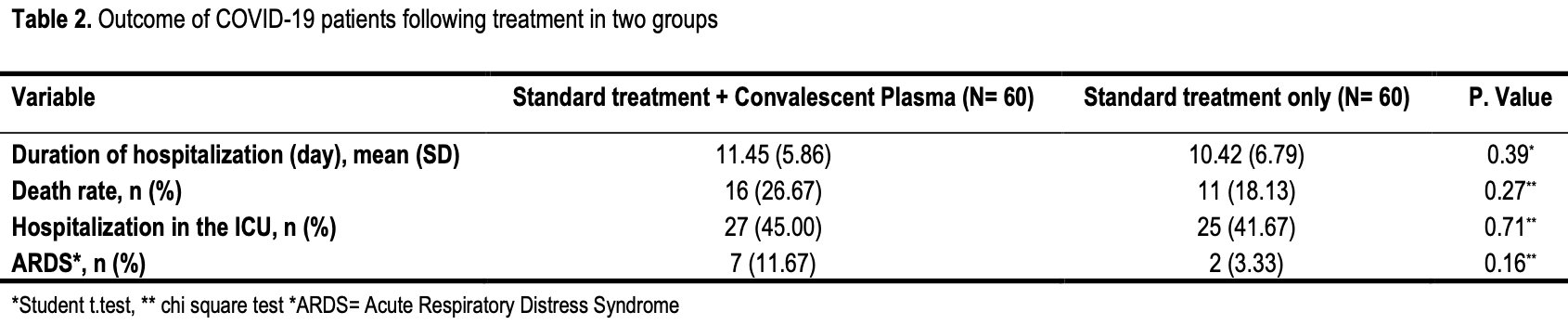
RCT 120 hospitalized patients in Iran, showing no significant differences with convalescent plasma treatment.
|
risk of death, 45.5% higher, RR 1.45, p = 0.38, treatment 16 of 60 (26.7%), control 11 of 60 (18.3%).
|
|
risk of ICU admission, 8.0% higher, RR 1.08, p = 0.85, treatment 27 of 60 (45.0%), control 25 of 60 (41.7%).
|
|
risk of ARDS, 250.0% higher, RR 3.50, p = 0.16, treatment 7 of 60 (11.7%), control 2 of 60 (3.3%).
|
|
hospitalization time, 9.9% higher, relative time 1.10, p = 0.39, treatment 60, control 60.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Jalili et al., 1 Jan 2022, Randomized Controlled Trial, Iran, peer-reviewed, 15 authors, study period May 2020 - July 2020.
Effect of Convalescent Plasma Therapy on Clinical Improvement of COVID-19 Patients: A Randomized Clinical Trial
Background: Due to the critical condition of COVID-19, it is necessary to evaluate the efficacy of administrating convalescent plasma to COVID-19 patients. Therefore, we decided to design a clinical trial to investigate the effect of convalescent plasma of patients recovered from COVID-19 on the treatment outcome of COVID-19-infected patients.
Materials and Methods: In this parallel randomized controlled clinical trial, patients in the intervention group received standard treatment plus convalescent plasma of patients recovered from COVID-19. We allocated 60 patients to each treatment group through balanced block randomization. Then, COVID-19 outcomes, vital signs, and biochemical parameters were compared between the two treatment groups by the independent t test and ANCOVA.
Results: The mean age (SD) of the patients in the intervention and standard treatment groups was 52.84 (15.77) and 55.15 (14.34) years, respectively. Although patients in the intervention group reported more hospitalization days (11.45±5.86 vs. 10.42±6.79), death rates (26.67% vs. 18.13%), ICU admission (45 vs. 41.67%), and ARDS (11.67% vs. 3.33%), these differences were not statistically significant (P>0.05). Moreover, the two groups were homogenous in vital signs and biochemical parameters before and after treatment (P>0.05).
Conclusion: The present study indicated that convalescent plasma therapy has no significant effect on the survival, hospitalization, and ICU admission of COVID-19 patients.
References
Abolghasemi, Eshghi, Cheraghali, Fooladi, Moghaddam et al., Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study, Transfus Apher Sci
Agarwal, Mukherjee, Kumar, Chatterjee, Bhatnagar et al., Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial), BMJ
Arabi, Balkhy, Hajeer, Bouchama, Hayden et al., Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol, Springerplus
Cheng, Wong, Soo, Wong, Lee et al., Use of convalescent plasma therapy in SARS patients in Hong Kong, Eur J Clin Microbiol Infect Dis
Duan, Liu, Li, Zhang, Yu et al., Effectiveness of convalescent plasma therapy in severe COVID-19 patients, Proc Natl Acad Sci U S A
Fleming, Raabe, Current studies of convalescent plasma therapy for COVID-19 may underestimate risk of antibodydependent enhancement, J Clin Virol
Gharbharan, Jordans, Geurtsvankessel, Hollander, Karim et al., Convalescent plasma for COVID-19. A randomized clinical trial
Hung, To, Lee, Lee, Chan et al., Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection, Clin Infect Dis
Joyner, Bruno, Klassen, Kunze, Johnson et al., Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients, Mayo Clin Proc
Lai, Treatment of severe acute respiratory syndrome, Eur J Clin Microbiol Infect Dis
Lai, Wang, Wang, Hsueh, Ko et al., Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status, Int J Antimicrob Agents
Langhi, Junior, Santis, Bordin, COVID-19 convalescent plasma transfusion, Hematol Transfus Cell Ther
Lee, Wheatley, Kent, Dekosky, Antibodydependent enhancement and SARS-CoV-2 vaccines and therapies, Nat Microbiol
Madariaga, Guthmiller, Schrantz, Jansen, Christensen et al., Clinical predictors of donor antibody titre and correlation with recipient antibody response in a COVID-19 convalescent plasma clinical trial, J Intern Med
Mair-Jenkins, Saavedra-Campos, Baillie, Cleary, Khaw et al., The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis, J Infect Dis
Salazar, Christensen, Graviss, Nguyen, Castillo et al., Treatment of Coronavirus Disease 2019 Patients with Convalescent Plasma Reveals a Signal of Significantly Decreased Mortality, Am J Pathol
Shen, Wang, Zhao, Yang, Li et al., Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma, JAMA
Soo, Cheng, Wong, Hui, Lee et al., Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients, Clin Microbiol Infect
Tanne, Covid-19: FDA approves use of convalescent plasma to treat critically ill patients, BMJ
To, Tsang, Leung, Tam, Wu et al., Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study, Lancet Infect Dis
Wu, Wang, Liu, Wang, Chen et al., Neutralizing Antibody Responses to SARS-CoV-2 in a COVID-19 Recovered Patient Cohort and Their Implications