Effect of timing of casirivimab and imdevimab administration relative to mRNA-1273 COVID-19 vaccination on vaccine-induced SARS-CoV-2 neutralising antibody responses: a prospective, open-label, phase 2, randomised controlled trial
et al., The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00421-3, NCT04852978, Sep 2024
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
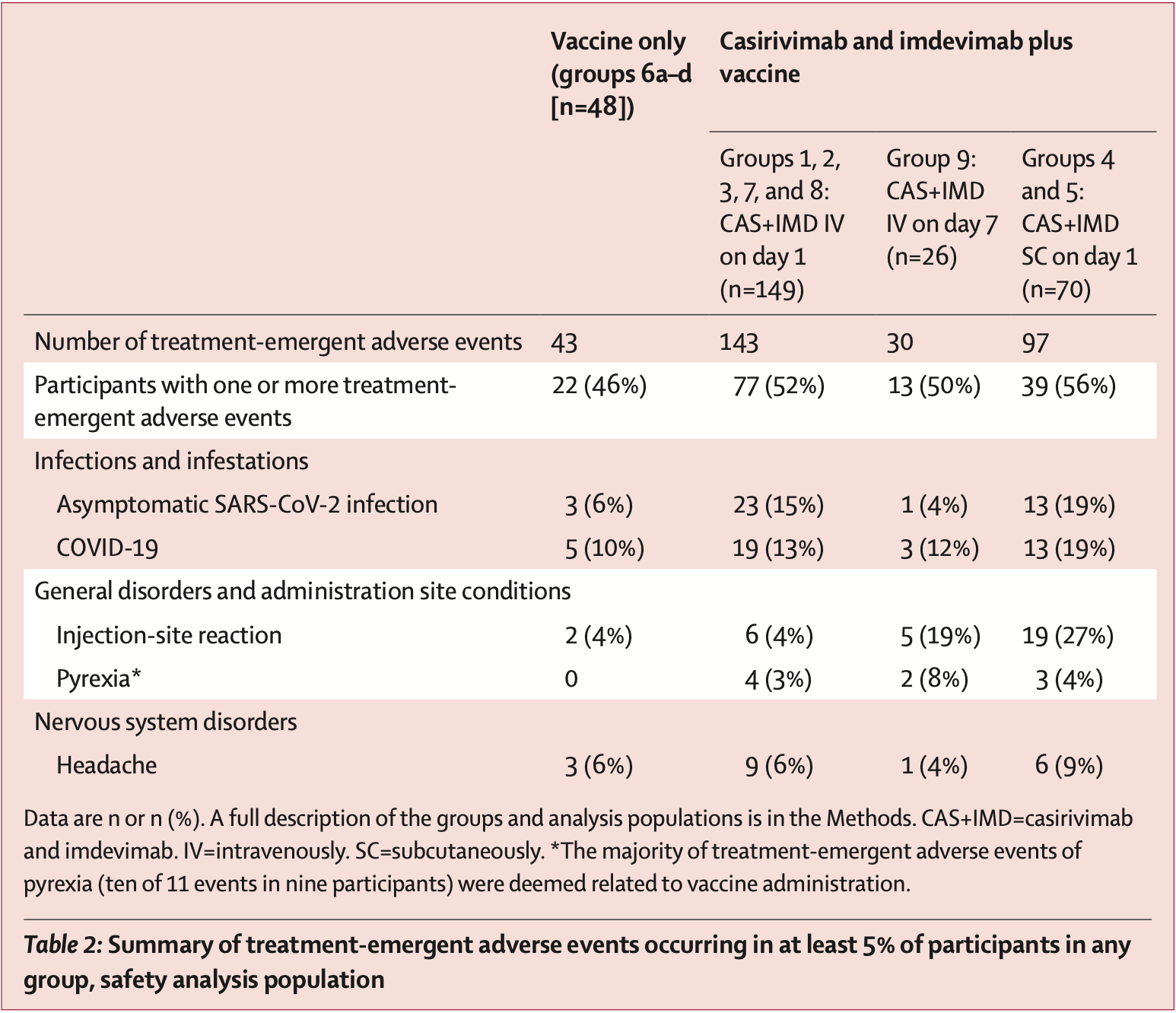
RCT 293 healthy adults focusing on the timing of casirivimab and imdevimab administration relative to mRNA-1273, but also showing the incidence of COVID-19 for each group, with higher incidence in the casirivimab and imdevimab groups (without statistical significance). Authors note the high prevalence of omicron variants which may explain the lack of efficacy seen.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of symptomatic case, 37.1% higher, RR 1.37, p = 0.65, treatment 35 of 245 (14.3%), control 5 of 48 (10.4%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Isa et al., 2 Sep 2024, Randomized Controlled Trial, USA, peer-reviewed, 40 authors, study period 29 April, 2021 - 21 November, 2022, trial NCT04852978 (history).
Contact: flonza.isa@regeneron.com.
Effect of timing of casirivimab and imdevimab administration relative to mRNA-1273 COVID-19 vaccination on vaccine-induced SARS-CoV-2 neutralising antibody responses: a prospective, open-label, phase 2, randomised controlled trial
The Lancet Infectious Diseases, doi:10.1016/s1473-3099(24)00421-3
Background Deeper insight is needed on how monoclonal antibodies (mAbs) affect vaccine-mediated immune responses when targeting the same protein. We describe the first prospective randomised trial designed to understand mAb-mediated alterations in vaccine-induced immune responses to SARS-CoV-2 spike protein epitopes. Methods This randomised, open-label, parallel-group study assessed the potential interaction of a mAb combination, casirivimab and imdevimab, with a vaccine, Moderna's mRNA-1273, in healthy SARS-CoV-2 immunologically naive, seronegative adults at six centres in the USA. Participants were randomly assigned (per prespecified randomisation ratios within enrolment waves) according to a computer-generated randomisation scheme, stratified by age (<65 years and ≥65 years), to various intravenous or subcutaneous doses of casirivimab and imdevimab before, after, or at the same time as mRNA-1273 or to mRNA-1273 only. The doses of casirivimab and imdevimab were chosen to mimic various time intervals between receipt of 1200 mg of the mAb and the first dose of a primary series with mRNA-1273. The primary endpoint was vaccine-induced 50% inhibitory dilution neutralising antibody titres to SARS-CoV-2 spike protein, 56 days after the first vaccination. Secondary endpoints included vaccine-induced total antibodies to SARS-CoV-2 antigens and incidence of treatment-emergent adverse events. Exploratory endpoints included blood-derived T-cell and B-cell responses. The per-protocol set was used for the analysis of the primary endpoint and included all randomly assigned participants who received both doses of the vaccine and completed the injection or infusion of casirivimab and imdevimab per protocol, had no evidence of SARS-CoV-2 infection in the past or in the 56 days after the first dose of vaccine, and did not receive any intervention outside of the study that could alter the immune response. Safety was assessed in the safety analysis set, which included all randomly assigned participants who had received one or more doses of mRNA-1273 or any study drug, and analysed based on treatment received. The study is registered with ClinicalTrials.gov, NCT04852978, and is complete. Findings Between April 29, 2021, and Nov 21, 2022, 807 participants were assessed for eligibility and 295 were randomly assigned. 293 participants were included in the safety analysis set and 260 were included in the per-protocol set. All vaccinated participants developed neutralising antibodies to SARS-CoV-2, with median titres above the published protective threshold (100 IU/mL) against the SARS-CoV-2 D614G variant (considered a reference strain at the time the initial COVID-19 vaccines were developed). Titres were decreased up to 4-fold (median titres 280-450 IU/mL for casirivimab and imdevimab vs 1160 IU/mL for vaccine only on day 56) when casirivimab and imdevimab was given 85 days or less before vaccination (150-1200 mg intravenously) or co-administered subcutaneously (600..
References
Anderson, Rouphael, Widge, Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults, N Engl J Med
Andrews, Stowe, Kirsebom, COVID-19 vaccine effectiveness against the omicron (B.1.1.529) variant, N Engl J Med
Atmar, Lyke, Deming, Homologous and heterologous COVID-19 booster vaccinations, N Engl J Med
Bates, Leier, Lyski, Neutralization of SARS-CoV-2 variants by convalescent and BNT162b2 vaccinated serum, Nat Commun
Benkeser, Montefiori, Mcdermott, Comparing antibody assays as correlates of protection against COVID-19 in the COVE mRNA-1273 vaccine efficacy trial, Sci Transl Med
Benschop, Tuttle, Zhang, The anti-SARS-CoV-2 monoclonal antibody bamlanivimab minimally affects the endogenous immune response to COVID-19 vaccination, Sci Transl Med
Cohen, Nirula, Mulligan, Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial, JAMA
Dougan, Nirula, Azizad, Bamlanivimab plus etesevimab in mild or moderate COVID-19, N Engl J Med
Feng, Phillips, White, Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection, Nat Med
Follmann, Brien, Fintzi, Examining protective effects of SARS-CoV-2 neutralizing antibodies after vaccination or monoclonal antibody administration, Nat Commun
Gilbert, Montefiori, Mcdermott, Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial, Science
Goel, Apostolidis, Painter, Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination, Sci Immunol
Greaney, Loes, Gentles, Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection, Sci Transl Med
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19, N Engl J Med
Hermens, Kesmir, Role of T cells in severe COVID-19 disease, protection, and long term immunity, Immunogenetics
Irvin, Ganguly, Weiss, REGEN-COV antibody cocktail bioanalytical strategy: comparison of LC-MRM-MS and immunoassay methods for drug quantification, Bioanalysis
Jackson, Anderson, Rouphael, An mRNA vaccine against SARS-CoV-2-preliminary report, N Engl J Med
Levin, Ustianowski, Wit, Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of COVID-19, N Engl J Med
Montgomery, Hobbs, Padilla, Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial, Lancet Respir Med
Nkolola, Yu, Wan, A bivalent SARS-CoV-2 monoclonal antibody combination does not affect the immunogenicity of a vector-based COVID-19 vaccine in macaques, Sci Transl Med
O'brien, Forleo-Neto, Musser, Subcutaneous REGEN-COV antibody combination to prevent COVID-19, N Engl J Med
O'brien, Forleo-Neto, Sarkar, Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: a randomized clinical trial, JAMA
O'brien, Forleo-Neto, Sarkar, Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: a randomized clinical trial, JAMA
Portal-Celhay, Forleo-Neto, Eagan, Virologic efficacy of casirivimab and imdevimab COVID-19 antibody combination in outpatients with SARS-CoV-2 infection: a phase 2 dose-ranging randomized clinical trial, JAMA Netw Open
Schaefer-Babajew, Wang, Muecksch, Antibody feedback regulates immune memory after SARS-CoV-2 mRNA vaccination, Nature
Weinreich, Sivapalasingam, Norton, REGEN-COV antibody combination and outcomes in outpatients with COVID-19, N Engl J Med
Zarnitsyna, Ellebedy, Davis, Jacob, Ahmed et al., Masking of antigenic epitopes by antibodies shapes the humoral immune response to influenza, Philos Trans R Soc Lond B Biol Sci
DOI record:
{
"DOI": "10.1016/s1473-3099(24)00421-3",
"ISSN": [
"1473-3099"
],
"URL": "http://dx.doi.org/10.1016/S1473-3099(24)00421-3",
"alternative-id": [
"S1473309924004213"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Effect of timing of casirivimab and imdevimab administration relative to mRNA-1273 COVID-19 vaccination on vaccine-induced SARS-CoV-2 neutralising antibody responses: a prospective, open-label, phase 2, randomised controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S1473-3099(24)00421-3"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S1473-3099(24)00559-0"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 Elsevier Ltd. All rights are reserved, including those for text and data mining, AI training, and similar technologies."
}
],
"author": [
{
"affiliation": [],
"family": "Isa",
"given": "Flonza",
"sequence": "first"
},
{
"affiliation": [],
"family": "Gonzalez Ortiz",
"given": "Ana M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meyer",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hamilton",
"given": "Jennifer D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Olenchock",
"given": "Benjamin A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brackin",
"given": "Taylor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ganguly",
"given": "Samit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Forleo-Neto",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Faria",
"given": "Lori",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heirman",
"given": "Ingeborg",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marovich",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hutter",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Polakowski",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Irvin",
"given": "Susan C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thakur",
"given": "Mazhar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hooper",
"given": "Andrea T",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baum",
"given": "Alina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Petro",
"given": "Christopher D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fakih",
"given": "Faisal A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McElrath",
"given": "M Juliana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Rosa",
"given": "Stephen C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cohen",
"given": "Kristen W",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Williams",
"given": "LaTonya D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hellman",
"given": "Caleb A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Odeh",
"given": "Ahmad J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Aloki H",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tomaras",
"given": "Georgia D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Geba",
"given": "Gregory P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kyratsous",
"given": "Christos A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Musser",
"given": "Bret",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yancopoulos",
"given": "George D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Herman",
"given": "Gary A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turner",
"given": "Kenneth C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kim",
"given": "Yunji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Konis",
"given": "George",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rosenthal",
"given": "Mark J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Trbovic",
"given": "Caryn F",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kowal",
"given": "Bari",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DiCioccio",
"given": "A Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dakin",
"given": "Paula",
"sequence": "additional"
}
],
"container-title": "The Lancet Infectious Diseases",
"container-title-short": "The Lancet Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"em-consulte.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
9,
2
]
],
"date-time": "2024-09-02T22:33:00Z",
"timestamp": 1725316380000
},
"deposited": {
"date-parts": [
[
2024,
9,
2
]
],
"date-time": "2024-09-02T22:33:16Z",
"timestamp": 1725316396000
},
"funder": [
{
"DOI": "10.13039/100009857",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100009857",
"id-type": "DOI"
}
],
"name": "Regeneron Pharmaceuticals Inc"
}
],
"indexed": {
"date-parts": [
[
2024,
9,
3
]
],
"date-time": "2024-09-03T00:20:38Z",
"timestamp": 1725322838941
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2024,
9
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
1
]
],
"date-time": "2024-09-01T00:00:00Z",
"timestamp": 1725148800000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
1
]
],
"date-time": "2024-09-01T00:00:00Z",
"timestamp": 1725148800000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
1
]
],
"date-time": "2024-09-01T00:00:00Z",
"timestamp": 1725148800000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
1
]
],
"date-time": "2024-09-01T00:00:00Z",
"timestamp": 1725148800000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
1
]
],
"date-time": "2024-09-01T00:00:00Z",
"timestamp": 1725148800000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
1
]
],
"date-time": "2024-09-01T00:00:00Z",
"timestamp": 1725148800000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
1
]
],
"date-time": "2024-09-01T00:00:00Z",
"timestamp": 1725148800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309924004213?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1473309924004213?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
9
]
]
},
"published-print": {
"date-parts": [
[
2024,
9
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV antibody combination and outcomes in outpatients with COVID-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "e81",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(24)00421-3_bib1",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2109682",
"article-title": "Subcutaneous REGEN-COV antibody combination to prevent COVID-19",
"author": "O'Brien",
"doi-asserted-by": "crossref",
"first-page": "1184",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(24)00421-3_bib2",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1126/scitranslmed.abi9915",
"article-title": "Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection",
"author": "Greaney",
"doi-asserted-by": "crossref",
"journal-title": "Sci Transl Med",
"key": "10.1016/S1473-3099(24)00421-3_bib3",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-25479-6",
"article-title": "Neutralization of SARS-CoV-2 variants by convalescent and BNT162b2 vaccinated serum",
"author": "Bates",
"doi-asserted-by": "crossref",
"journal-title": "Nat Commun",
"key": "10.1016/S1473-3099(24)00421-3_bib4",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(24)00421-3_bib5",
"volume": "386",
"year": "2022"
},
{
"key": "10.1016/S1473-3099(24)00421-3_bib6",
"series-title": "Fact sheet for health care providers: Emergency Use Authorization (EUA) of REGEN-COV (casirivimab and imdevimab)",
"year": "2021"
},
{
"key": "10.1016/S1473-3099(24)00421-3_bib7",
"series-title": "Fact sheet for health care providers: Emergency Use Authorization (EUA) of bamlanivimab and etesevimab",
"year": "2022"
},
{
"key": "10.1016/S1473-3099(24)00421-3_bib8",
"series-title": "Fact sheet for healthcare providers: Emergency Use Authorization for Evusheld (tixagevimab co-packaged with cilgavimab)",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01540-1",
"article-title": "Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection",
"author": "Feng",
"doi-asserted-by": "crossref",
"first-page": "2032",
"journal-title": "Nat Med",
"key": "10.1016/S1473-3099(24)00421-3_bib9",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1038/s41467-023-39292-w",
"article-title": "Examining protective effects of SARS-CoV-2 neutralizing antibodies after vaccination or monoclonal antibody administration",
"author": "Follmann",
"doi-asserted-by": "crossref",
"journal-title": "Nat Commun",
"key": "10.1016/S1473-3099(24)00421-3_bib10",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1126/scitranslmed.ade9078",
"article-title": "Comparing antibody assays as correlates of protection against COVID-19 in the COVE mRNA-1273 vaccine efficacy trial",
"author": "Benkeser",
"doi-asserted-by": "crossref",
"journal-title": "Sci Transl Med",
"key": "10.1016/S1473-3099(24)00421-3_bib11",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2116620",
"article-title": "Intramuscular AZD7442 (tixagevimab–cilgavimab) for prevention of COVID-19",
"author": "Levin",
"doi-asserted-by": "crossref",
"first-page": "2188",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(24)00421-3_bib12",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.24939",
"article-title": "Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: a randomized clinical trial",
"author": "O'Brien",
"doi-asserted-by": "crossref",
"first-page": "432",
"journal-title": "JAMA",
"key": "10.1016/S1473-3099(24)00421-3_bib13",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(22)00180-1",
"article-title": "Efficacy and safety of intramuscular administration of tixagevimab–cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial",
"author": "Montgomery",
"doi-asserted-by": "crossref",
"first-page": "985",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S1473-3099(24)00421-3_bib14",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab plus etesevimab in mild or moderate COVID-19",
"author": "Dougan",
"doi-asserted-by": "crossref",
"first-page": "1382",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(24)00421-3_bib15",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.8828",
"article-title": "Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial",
"author": "Cohen",
"doi-asserted-by": "crossref",
"first-page": "46",
"journal-title": "JAMA",
"key": "10.1016/S1473-3099(24)00421-3_bib16",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1098/rstb.2014.0248",
"article-title": "Masking of antigenic epitopes by antibodies shapes the humoral immune response to influenza",
"author": "Zarnitsyna",
"doi-asserted-by": "crossref",
"journal-title": "Philos Trans R Soc Lond B Biol Sci",
"key": "10.1016/S1473-3099(24)00421-3_bib17",
"volume": "370",
"year": "2015"
},
{
"DOI": "10.1126/scitranslmed.abn3041",
"article-title": "The anti-SARS-CoV-2 monoclonal antibody bamlanivimab minimally affects the endogenous immune response to COVID-19 vaccination",
"author": "Benschop",
"doi-asserted-by": "crossref",
"journal-title": "Sci Transl Med",
"key": "10.1016/S1473-3099(24)00421-3_bib19",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1126/scitranslmed.abo6160",
"article-title": "A bivalent SARS-CoV-2 monoclonal antibody combination does not affect the immunogenicity of a vector-based COVID-19 vaccine in macaques",
"author": "Nkolola",
"doi-asserted-by": "crossref",
"journal-title": "Sci Transl Med",
"key": "10.1016/S1473-3099(24)00421-3_bib20",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-05609-w",
"article-title": "Antibody feedback regulates immune memory after SARS-CoV-2 mRNA vaccination",
"author": "Schaefer-Babajew",
"doi-asserted-by": "crossref",
"first-page": "735",
"journal-title": "Nature",
"key": "10.1016/S1473-3099(24)00421-3_bib21",
"volume": "613",
"year": "2023"
},
{
"key": "10.1016/S1473-3099(24)00421-3_bib22",
"series-title": "Emergency Use Authorization 091",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2022.25411",
"article-title": "Virologic efficacy of casirivimab and imdevimab COVID-19 antibody combination in outpatients with SARS-CoV-2 infection: a phase 2 dose-ranging randomized clinical trial",
"author": "Portal-Celhay",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/S1473-3099(24)00421-3_bib23",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.4155/bio-2021-0190",
"article-title": "REGEN-COV antibody cocktail bioanalytical strategy: comparison of LC-MRM-MS and immunoassay methods for drug quantification",
"author": "Irvin",
"doi-asserted-by": "crossref",
"first-page": "1827",
"journal-title": "Bioanalysis",
"key": "10.1016/S1473-3099(24)00421-3_bib24",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2028436",
"article-title": "Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults",
"author": "Anderson",
"doi-asserted-by": "crossref",
"first-page": "2427",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(24)00421-3_bib25",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2022483",
"article-title": "An mRNA vaccine against SARS-CoV-2—preliminary report",
"author": "Jackson",
"doi-asserted-by": "crossref",
"first-page": "1920",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(24)00421-3_bib26",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1126/science.abm3425",
"article-title": "Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial",
"author": "Gilbert",
"doi-asserted-by": "crossref",
"first-page": "43",
"journal-title": "Science",
"key": "10.1016/S1473-3099(24)00421-3_bib27",
"volume": "375",
"year": "2022"
},
{
"DOI": "10.1126/sciimmunol.abi6950",
"article-title": "Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination",
"author": "Goel",
"doi-asserted-by": "crossref",
"journal-title": "Sci Immunol",
"key": "10.1016/S1473-3099(24)00421-3_bib28",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116414",
"article-title": "Homologous and heterologous COVID-19 booster vaccinations",
"author": "Atmar",
"doi-asserted-by": "crossref",
"first-page": "1046",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(24)00421-3_bib29",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1007/s00251-023-01294-9",
"article-title": "Role of T cells in severe COVID-19 disease, protection, and long term immunity",
"author": "Hermens",
"doi-asserted-by": "crossref",
"first-page": "295",
"journal-title": "Immunogenetics",
"key": "10.1016/S1473-3099(24)00421-3_bib30",
"volume": "75",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2119451",
"article-title": "COVID-19 vaccine effectiveness against the omicron (B.1.1.529) variant",
"author": "Andrews",
"doi-asserted-by": "crossref",
"first-page": "1532",
"journal-title": "N Engl J Med",
"key": "10.1016/S1473-3099(24)00421-3_bib31",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.24939",
"article-title": "Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: a randomized clinical trial",
"author": "O'Brien",
"doi-asserted-by": "crossref",
"first-page": "432",
"journal-title": "JAMA",
"key": "10.1016/S1473-3099(24)00421-3_bib32",
"volume": "327",
"year": "2022"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1473309924004213"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effect of timing of casirivimab and imdevimab administration relative to mRNA-1273 COVID-19 vaccination on vaccine-induced SARS-CoV-2 neutralising antibody responses: a prospective, open-label, phase 2, randomised controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}
