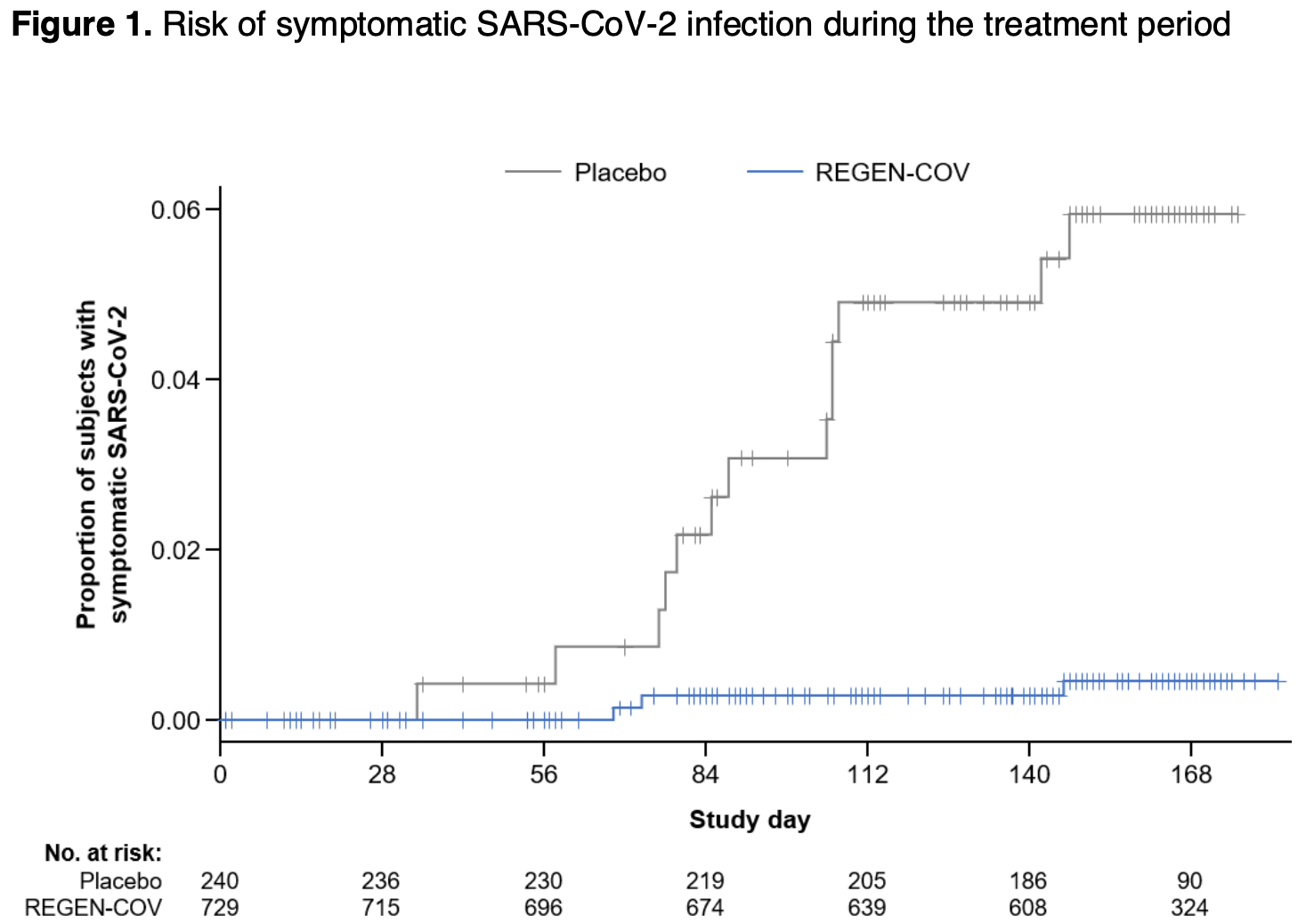
Repeat Subcutaneous Administration of REGEN-COV® in Adults is Well-Tolerated and Prevents the Occurrence of COVID-19
et al., medRxiv, doi:10.1101/2021.11.10.21265889, NCT04519437, Nov 2021
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 969 patients, 729 treated with monthly subcutaneous casirivimab/imdevimab, showing significantly lower risk of COVID-19 with treatment. There were no grade 3 injection site reactions or hypersensitivity reactions. Slightly more TEAEs were reported with treatment (54.9% vs. 48.3%), due to grade 1-2 ISRs. Serious adverse events were rare and occurred with similar percentages for treatment and control groups. There were no deaths. NCT04519437 (history).
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of symptomatic case, 92.6% lower, RR 0.07, p = 0.002, treatment 3 of 729 (0.4%), control 13 of 240 (5.4%), NNT 20, odds ratio converted to relative risk.
|
|
risk of case, 92.7% lower, RR 0.07, p = 0.002, treatment 0 of 729 (0.0%), control 10 of 240 (4.2%), NNT 24, odds ratio converted to relative risk, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), seroconversion.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Conflicts of interest:
employee of the drug patent holder.
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Isa et al., 16 Nov 2021, Double Blind Randomized Controlled Trial, USA, preprint, 31 authors, study period 26 July, 2020 - 21 May, 2021, trial NCT04519437 (history).
Contact: flonza.isa@regeneron.com.
Repeat Subcutaneous Administration of REGEN-COV® in Adults is Well-Tolerated and Prevents the Occurrence of COVID-19
doi:10.1101/2021.11.10.21265889
Background: Data show that a single dose of casirivimab and imdevimab (REGEN-COV ® ) is effective in treating hospitalized individuals and outpatients with COVID-19 and in post-exposure prophylaxis. We present results from a phase 1, double-blind, placebo-controlled trial evaluating the safety, tolerability, and efficacy of repeat monthly doses of subcutaneous (SC) REGEN-COV in uninfected adult volunteers who were healthy or had chronic stable medical conditions. Methods: Subjects were randomized (3:1) to SC REGEN-COV 1200 mg or placebo dosed every 4 weeks for up to 6 doses. The primary and secondary endpoints evaluated the safety, pharmacokinetics, and immunogenicity of multiple-dose administration of REGEN-COV. Efficacy was evaluated by the incidence of COVID-19 or SARS-CoV-2 seroconversion.
Results: In total, 969 subjects were treated. Repeat monthly dosing of SC REGEN-COV led to a 92.4% relative risk reduction in clinically-defined COVID-19 compared to placebo (3/729 [0.4%] vs 13/240 [5.4%]; odds ratio: 0.07 [95% CI, 0.01-0.27]), and a 100% reduction in laboratory-confirmed COVID-19 (0/729 vs 10/240 [4.2%]; odds ratio 0.00). Development of anti-drug antibodies was low (<5% subjects). No grade ≥3 injection-site reactions (ISRs) or hypersensitivity reactions were reported. A slightly higher percentage of subjects reported TEAEs with REGEN-COV (54.9%) than placebo (48.3%), due to ISRs (all grade 1-2). Serious adverse events were rare and occurred at similar percentages in the REGEN-COV and placebo groups. No deaths were reported in the 6-month treatment period.
Notes
References
Avanzato, Matson, Seifert, Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer, Cell
Baden, Sahly, Essink, Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine, N Engl J Med
Baum, Fulton, Wloga, Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies, Science
Baum, Kyratsous, SARS-CoV-2 spike therapeutic antibodies in the age of variants, J Exp Med
Bird, Panopoulou, Shea, Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma, Lancet Haematol
Copin, Baum, Wloga, The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies, Cell
Hansen, Baum, Pascal, Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail, Science
Harpaz, Dahl, Dooling, Prevalence of Immunosuppression Among US Adults, JAMA
Horby, Mafham, Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Kuritzkes, Bamlanivimab for Prevention of COVID-19, JAMA
Lontok, How Effective Are COVID-19 Vaccines in Immunocompromised People?, American Society for Microbiology
Ludwig, Zarbock, Coronaviruses and SARS-CoV-2: a brief overview
Munro, Covid-19: 40% of patients with weakened immune system mount lower response to vaccines, BMJ
O'brien, Forleo-Neto, Musser, Subcutaneous REGEN-COV antibody combination to prevent Covid-19, N Engl J Med
Polack, Thomas, Kitchin, Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine, N Engl J Med
Rosenthal, Cao, Gundrum, Sianis, Safo, Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19, JAMA Netw Open
Tenforde, Olson, Self, Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged >/=65 years -United States, January-March 2021, MMWR Morb Mortal Wkly Rep
Voysey, Clemens, Madhi, Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, Lancet
Wallace, Kenney, Malani, Clauw, Nallamothu et al., Prevalence of Immunosuppressive Drug Use Among Commercially Insured US Adults, 2018-2019, JAMA Netw Open
Wang, Hu, Hu, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA
Weinreich, Sivapalasingam, Norton, REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med
Who Director, at the media briefing on COVID-19
Williamson, Walker, Bhaskaran, Factors associated with COVID-19-related death using OpenSAFELY, Nature
Wu, Zhao, Yu, Author correction: A new coronavirus associated with human respiratory disease in China, Nature
Zhu, Zhang, Wang, A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med
DOI record:
{
"DOI": "10.1101/2021.11.10.21265889",
"URL": "http://dx.doi.org/10.1101/2021.11.10.21265889",
"abstract": "<jats:p><jats:bold>Background:</jats:bold> Data show that a single dose of casirivimab and imdevimab (REGEN-COV®) is effective in treating hospitalized individuals and outpatients with COVID-19 and in post-exposure prophylaxis. We present results from a phase 1, double-blind, placebo-controlled trial evaluating the safety, tolerability, and efficacy of repeat monthly doses of subcutaneous (SC) REGEN-COV in uninfected adult volunteers who were healthy or had chronic stable medical conditions.\n\n<jats:bold>Methods:</jats:bold> Subjects were randomized (3:1) to SC REGEN-COV 1200 mg or placebo dosed every 4 weeks for up to 6 doses. The primary and secondary endpoints evaluated the safety, pharmacokinetics, and immunogenicity of multiple-dose administration of REGEN-COV. Efficacy was evaluated by the incidence of COVID-19 or SARS-CoV-2 seroconversion.\n\n<jats:bold>Results:</jats:bold> In total, 969 subjects were treated. Repeat monthly dosing of SC REGEN-COV led to a 92.4% relative risk reduction in clinically-defined COVID-19 compared to placebo (3/729 [0.4%] vs 13/240 [5.4%]; odds ratio: 0.07 [95% CI, 0.01–0.27]), and a 100% reduction in laboratory-confirmed COVID-19 (0/729 vs 10/240 [4.2%]; odds ratio 0.00). Development of anti-drug antibodies was low (<5% subjects). No grade ≥3 injection-site reactions (ISRs) or hypersensitivity reactions were reported. A slightly higher percentage of subjects reported TEAEs with REGEN-COV (54.9%) than placebo (48.3%), due to ISRs (all grade 1-2). Serious adverse events were rare and occurred at similar percentages in the REGEN-COV and placebo groups. No deaths were reported in the 6-month treatment period. \n\n<jats:bold>Conclusions:</jats:bold> Repeated monthly administration of 1200 mg SC REGEN-COV was well-tolerated with low immunogenicity, and showed a substantial risk reduction in COVID-19 occurrence.\n\n(ClinicalTrials.gov identifier, <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04519437\">NCT04519437</jats:ext-link>)</jats:p>",
"accepted": {
"date-parts": [
[
2021,
11,
16
]
]
},
"author": [
{
"affiliation": [],
"family": "Isa",
"given": "Flonza",
"sequence": "first"
},
{
"affiliation": [],
"family": "Forleo-Neto",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meyer",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Wenjun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rasmussen",
"given": "Scott",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Armas",
"given": "Danielle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oshita",
"given": "Masaru",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brinson",
"given": "Cynthia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Folkerth",
"given": "Steven",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Faria",
"given": "Lori",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heirman",
"given": "Ingeborg",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sarkar",
"given": "Neena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Musser",
"given": "Bret J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bansal",
"given": "Shikha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "O'Brien",
"given": "Meagan P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turner",
"given": "Kenneth C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ganguly",
"given": "Samit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahmood",
"given": "Adnan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dupljak",
"given": "Ajla",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hooper",
"given": "Andrea T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hamilton",
"given": "Jennifer D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kim",
"given": "Yunji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kowal",
"given": "Bari",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soo",
"given": "Yuhwen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Geba",
"given": "Gregory P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lipsich",
"given": "Leah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Braunstein",
"given": "Ned",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yancopoulos",
"given": "George D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Weinreich",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Herman",
"given": "Gary A.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "the COVID-19 Multi-dose Trial Team",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
11,
16
]
],
"date-time": "2021-11-16T16:56:14Z",
"timestamp": 1637081774000
},
"deposited": {
"date-parts": [
[
2021,
11,
16
]
],
"date-time": "2021-11-16T16:56:14Z",
"timestamp": 1637081774000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2021,
11,
16
]
],
"date-time": "2021-11-16T17:13:56Z",
"timestamp": 1637082836908
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2021,
11,
16
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.11.10.21265889",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
11,
16
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
11,
16
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"Repeat Subcutaneous Administration of REGEN-COV® in Adults is Well-Tolerated and Prevents the Occurrence of COVID-19"
],
"type": "posted-content"
}
