Invivyd announces FDA authorization for emergency use of Pemgarda™ (formerly VYD222) for pre-exposure prophylaxis (PrEP) of COVID-19
, Press Release, 3/22, NCT06039449, Mar 2024
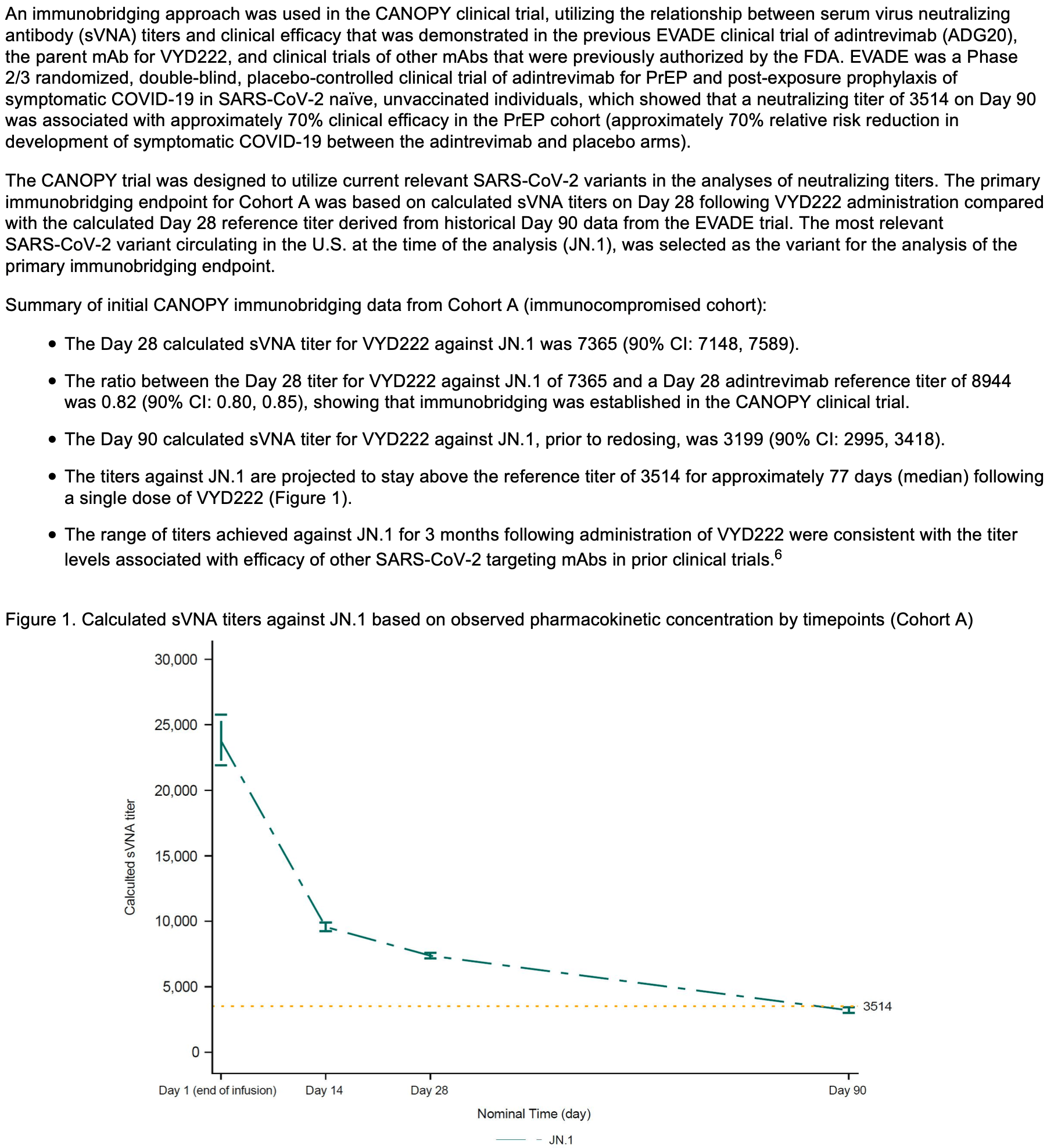
RCT 623 patients reporting immunobridging results from cohort A with 306 immunocompromised patients. Immunobridging estimates efficacy from the relationship between serum virus neutralizing antibody titers and clinical efficacy demonstrated in previous trials. Calculated titers against the JN.1 variant were consistent with levels associated with efficacy in prior trials. Pemivibart received emergency use authorization from the FDA for pre-exposure prophylaxis in certain immunocompromised patients based on this data along with safety data.
Efficacy is variant dependent. In Vitro research shows reduced efficacy against KP.3.1.1, KP.1.1, LB.1, KP.3.3, and XEC variants1-4.
1.
Xie et al., Molecular Basis of High-Blood-Pressure-Enhanced and High-Fever-Temperature-Weakened Receptor-Binding Domain/Peptidase Domain Binding: A Molecular Dynamics Simulation Study, International Journal of Molecular Sciences, doi:10.3390/ijms26073250.
2.
Wang et al., Activity of Research-Grade Pemivibart against Recent SARS-CoV-2 JN.1 Sublineages, New England Journal of Medicine, doi:10.1056/NEJMc2410203.
Invivyd et al., 22 Mar 2024, Randomized Controlled Trial, USA, preprint, 1 author, trial NCT06039449 (history).
Invivyd Announces FDA Authorization for Emergency Use of PEMGARDA™ (Formerly VYD222) for Pre-exposure Prophylaxis (PrEP) of COVID-19
PEMGARDA (pemivibart) is authorized in the U.S. for PrEP of COVID-19 in certain adults and adolescents with moderateto-severe immune compromise Emergency use authorization based on positive immunobridging data and on safety data from the CANOPY clinical trial along with ongoing in vitro neutralizing activity against major SARS-CoV-2 variants, including JN.1 PEMGARDA is the first PrEP monoclonal antibody (mAb) to receive EUA from the U.S. FDA based on a novel, rapid, repeatable immunobridging trial design PEMGARDA is the first authorized mAb from Invivyd's novel technology platform approach designed to address the challenge of rapid viral evolution Product availability in the U.S. anticipated imminently Estimated cash and cash equivalents of $200.6 million as of December 31, 2023 In February 2024, the Company sold shares totaling $40.5 million in gross proceeds under its At-the-Market facility further strengthening its balance sheet ahead of PEMGARDA launch Conference call today at 4pm ET to discuss the EUA and commercial launch of PEMGARDA WALTHAM, Mass., March 22, 2024 (GLOBE NEWSWIRE) --Invivyd, Inc. (Nasdaq: IVVD), a biopharmaceutical company on a mission to protect the vulnerable from serious viral infectious diseases, today announced that PEMGARDA™ (pemivibart), formerly VYD222, a half-life extended monoclonal antibody (mAb), has received emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the pre-exposure prophylaxis (prevention) of COVID-19 in adults and adolescents (12 years of age and older weighing at least 40 kg) who have moderate-to-severe immune compromise due to certain medical conditions or receipt of certain immunosuppressive medications or treatments and are unlikely to mount an adequate immune response to COVID-19 vaccination. Recipients should not be currently infected with or have had a known recent exposure to an individual infected with SARS-CoV-2. "The PEMGARDA EUA marks a transformational moment for Invivyd and for the many moderately to severely immunocompromised people who are vulnerable to COVID-19 disease in the U.S. This EUA milestone represents strategic proofof-concept for our company and platform, affirming the unique strategy we embarked on over a year ago: to use rapid innovation and surrogate markers to bring new antibodies to market repeatedly," said Dave Hering, Chief Executive Officer of Invivyd. "PEMGARDA is the first authorized monoclonal antibody from our proprietary platform approach. We are committed to ongoing process improvement while working with global regulatory agencies with the aim to increase the speed and efficiency of new mAb candidate development even further. Additionally, we are planning to explore the protective clinical benefits of mAb prophylaxis for symptomatic COVID-19 disease in future studies."
References
Evans, Impact of COVID-19 on immunocompromised populations during the Omicron era: insights from the observational population-based INFORM study, The Lancet regional health. Europe
Schmidt, Antibody-mediated protection against symptomatic COVID-19 can be achieved at low serum neutralizing titers, Sci. Transl. Med
Singson, Robert, Factors Associated with Severe Outcomes Among Immunocompromised Adults Hospitalized for COVID-19 -COVID-NET, 10 States, March 2020-February 2022, MMWR. Morbidity and mortality weekly report
Stadler, Monoclonal antibody levels and protection from COVID-19, Nature communications, doi:10.1038/s41467-023-40204-1