Association between Nafamostat Mesylate and In-Hospital Mortality in Patients with Coronavirus Disease 2019: A Multicenter Observational Study
et al., Journal of Clinical Medicine, doi:10.3390/jcm11010116, Dec 2021
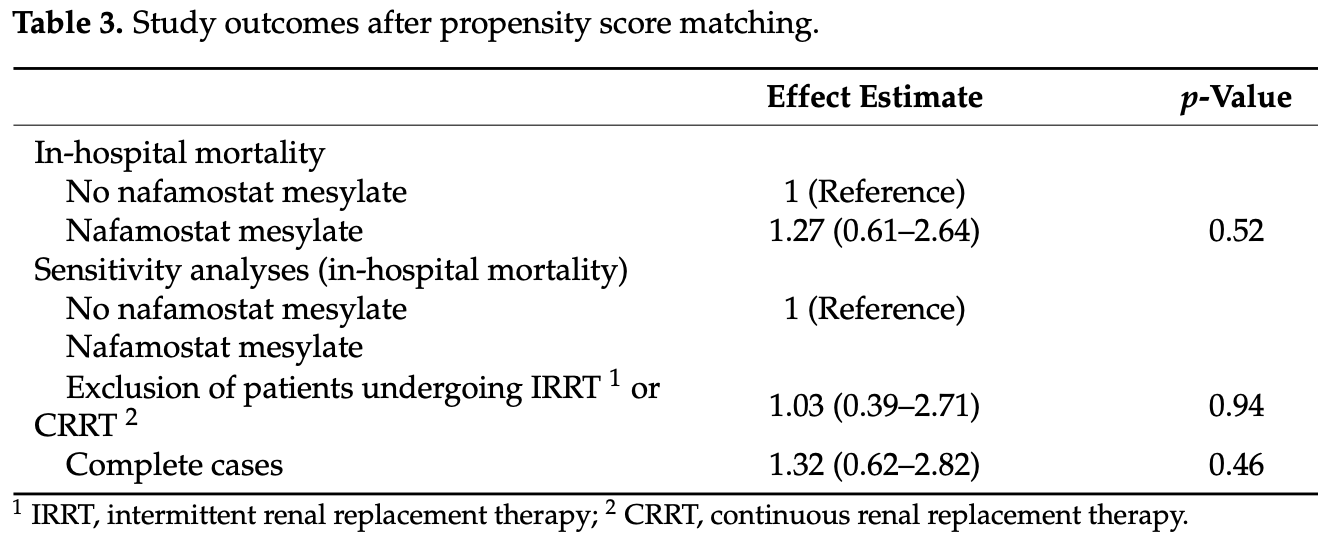
Retrospective multicenter observational study of 15,859 hospitalized COVID-19 patients in Japan showing no significant difference in in-hospital mortality with nafamostat mesylate. Very few patients received treatment and they had more severe disease on average. There may be significant residual confounding by indication.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers TMPRSS2 inhibitors and nafamostat.
|
risk of death, 27.0% higher, OR 1.27, p = 0.52, treatment 121, control 15,738, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Inokuchi et al., 26 Dec 2021, retrospective, Japan, peer-reviewed, 11 authors, study period 1 January, 2020 - 31 December, 2020.
Contact: iwagami-tky@umin.ac.jp (corresponding author), inokuchir-icu@md.tsukuba.ac.jp, jun.komi33@gmail.com, abetoshi111@gmail.com, ishimaru.miho.kf@u.tsukuba.ac.jp, adomimotohiko@gmail.com, ntamiya@md.tsukuba.ac.jp, tkuno@montefiore.org, taniguchi.yuta.ma@alumni.tsukuba.ac.jp, udakazuaki-tky@umin.ac.jp, ymiyamoto70@gmail.com.
Association between Nafamostat Mesylate and In-Hospital Mortality in Patients with Coronavirus Disease 2019: A Multicenter Observational Study
Journal of Clinical Medicine, doi:10.3390/jcm11010116
Nafamostat mesylate may be effective against coronavirus disease 2019 (COVID-19). However, it is not known whether its use is associated with reduced in-hospital mortality in clinical practice. We conducted a retrospective observational study to evaluate the effect of nafamostat mesylate in patients with COVID-19 using the Medical Data Vision Co. Ltd. hospital-based database in Japan. We compared patients with COVID-19 who were (n = 121) and were not (n = 15,738) administered nafamostat mesylate within 2 days of admission between January and December 2020. We conducted a 1:4 propensity score matching with multiple imputations for smoking status and body mass index and combined the 20 imputed propensity score-matched datasets to obtain the adjusted odds ratio for in-hospital mortality. Crude in-hospital mortality was 13.2% (16/121) and 5.0% (790/15,738), respectively. In the propensity score-matched analysis with multiple imputations, the adjusted odds ratio (use vs. no use of nafamostat mesylate) for in-hospital mortality was 1.27 (95% confidence interval: 0.61-2.64; p = 0.52). Sensitivity analyses showed similar results. The results of this retrospective observational study did not support an association between nafamostat mesylate and improved in-hospital outcomes in patients with COVID-19, although further studies with larger sample sizes are warranted to assess the generalizability of our findings.
Conflicts of Interest: The authors declare no conflict of interest.
References
Abduljabbar, Clinical efficacy of Nafamostat Mesylate in combination with Favipiravir for COVID-19 pneumonia treatment review article, Ann. Med. Surg, doi:10.1016/j.amsu.2021.102560
Aloisio, Micali, Swanson, Field, Horton, Analysis of Partially Observed Clustered Data using Generalized Estimating Equations and Multiple Imputation, Stata Journal: Promot. Commun. Stat. Stata, doi:10.1177/1536867X1401400410
Attah, Fagbemi, Olubiyi, Dada-Adegbola, Oluwadotun et al., Therapeutic Potentials of Antiviral Plants Used in Traditional African Medicine With COVID-19 in Focus: A Nigerian Perspective, Front. Pharmacol, doi:10.3389/fphar.2021.596855
Austin, An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies, Multivar. Behav. Res, doi:10.1080/00273171.2011.568786
Austin, Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensityscore matched samples, Stat. Med, doi:10.1002/sim.3697
Brookhart, Schneeweiss, Rothman, Glynn, Avorn et al., Variable Selection for Propensity Score Models, Am. J. Epidemiol, doi:10.1093/aje/kwj149
Cho, Han, Jeong, Park, Shim, A Novel Computational Approach for the Discovery of Drug Delivery System Candidates for COVID-19, Int. J. Mol. Sci, doi:10.3390/ijms22062815
Doi, Nishida, Shigematsu, Sadahiro, Itami et al., The Japanese Clinical Practice Guideline for acute kidney injury 2016, J. Intensiv. Care, doi:10.1186/s40560-018-0308-6
Doi, The, Group; Ikeda, Hayase, Moriya et al., Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with COVID-19: A case series, Crit. Care, doi:10.1186/s13054-020-03078-z
Hoffmann, Schroeder, Kleine-Weber, Müller, Drosten et al., Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19, Antimicrob. Agents Chemother, doi:10.1128/AAC.00754-20
Iwasaka, Shono, Tokuda, Nakashima, Yamamoto et al., Clinical improvement in a patient with severe coronavirus disease 2019 after administration of hydroxychloroquine and continuous hemodiafiltlation with nafamostat mesylate, J. Infect. Chemother, doi:10.1016/j.jiac.2020.08.001
Jang, Rhee, Three cases of treatment with nafamostat in elderly patients with COVID-19 pneumonia who need oxygen therapy, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.05.072
Jordan, Adab, Cheng, COVID-19: Risk factors for severe disease and death, BMJ, doi:10.1136/bmj.m1198
Kim, Garg, O'halloran, Whitaker, Pham et al., Risk Factors for Intensive Care Unit Admission and In-hospital Mortality Among Hospitalized Adults Identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET), Clin. Infect. Dis, doi:10.1093/cid/ciaa1012
Matsunaga, Hayakawa, Terada, Ohtsu, Asai et al., Clinical epidemiology of hospitalized patients with COVID-19 in Japan: Report of the COVID-19 REGISTRY JAPAN, Clin. Infect. Dis, doi:10.1093/cid/ciaa1470
Ono, Wada, Takahara, Shirotani, Indications for Computed Tomography in Patients With Mild Head Injury, Neurol. Med.-Chir, doi:10.2176/nmc.47.291
Quan, Li, Couris, Fushimi, Graham et al., Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries, Am. J. Epidemiol, doi:10.1093/aje/kwq433
Rosenbaum, Rubin, Constructing a Control Group Using Multivariate Matched Sampling Methods That Incorporate the Propensity Score, Am. Stat
Rubin, Propensity score methods, Am. J. Ophthalmol, doi:10.1016/j.ajo.2009.08.024
Rubin, Schenker, Multiple imputation in health-care databases: An overview and some applications, Stat. Med, doi:10.1002/sim.4780100410
Sallam, COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates, Vaccines, doi:10.3390/vaccines9020160
Suissa, Immortal time bias in pharmaco-epidemiology, Am. J. Epidemiol, doi:10.1093/aje/kwm324
Takahashi, Yoneda, Koba, Ueda, Tsuji et al., Potential mechanisms of nafamostat therapy for severe COVID-19 pneumonia with disseminated intravascular coagulation, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.10.093
Who, Update to living WHO guideline on drugs for COVID-19, BMJ
Yamamoto, Kiso, Sakai-Tagawa, Iwatsuki-Horimoto, Imai et al., The Anticoagulant Nafamostat Potently Inhibits SARS-CoV-2 S Protein-Mediated Fusion in a Cell Fusion Assay System and Viral Infection In Vitro in a Cell-Type-Dependent Manner, Viruses, doi:10.3390/v12060629
Yamana, Moriwaki, Horiguchi, Kodan, Fushimi et al., Validity of diagnoses, procedures, and laboratory data in Japanese administrative data, J. Epidemiology, doi:10.1016/j.je.2016.09.009
Zhang, Penninger, Li, Zhong, Slutsky, Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target, Intensive Care Med, doi:10.1007/s00134-020-05985-9
Zhuravel, Khmelnitskiy, Burlaka, Gritsan, Goloshchekin et al., Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: A randomised Phase II clinical trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.101169
DOI record:
{
"DOI": "10.3390/jcm11010116",
"ISSN": [
"2077-0383"
],
"URL": "http://dx.doi.org/10.3390/jcm11010116",
"abstract": "<jats:p>Nafamostat mesylate may be effective against coronavirus disease 2019 (COVID-19). However, it is not known whether its use is associated with reduced in-hospital mortality in clinical practice. We conducted a retrospective observational study to evaluate the effect of nafamostat mesylate in patients with COVID-19 using the Medical Data Vision Co. Ltd. hospital-based database in Japan. We compared patients with COVID-19 who were (n = 121) and were not (n = 15,738) administered nafamostat mesylate within 2 days of admission between January and December 2020. We conducted a 1:4 propensity score matching with multiple imputations for smoking status and body mass index and combined the 20 imputed propensity score-matched datasets to obtain the adjusted odds ratio for in-hospital mortality. Crude in-hospital mortality was 13.2% (16/121) and 5.0% (790/15,738), respectively. In the propensity score-matched analysis with multiple imputations, the adjusted odds ratio (use vs. no use of nafamostat mesylate) for in-hospital mortality was 1.27 (95% confidence interval: 0.61–2.64; p = 0.52). Sensitivity analyses showed similar results. The results of this retrospective observational study did not support an association between nafamostat mesylate and improved in-hospital outcomes in patients with COVID-19, although further studies with larger sample sizes are warranted to assess the generalizability of our findings.</jats:p>",
"alternative-id": [
"jcm11010116"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0001-6343-2298",
"affiliation": [
{
"name": "Department of Health Services Research, Faculty of Medicine, University of Tsukuba, Tsukuba 305-8575, Japan"
}
],
"authenticated-orcid": false,
"family": "Inokuchi",
"given": "Ryota",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Montefiore Medical Center, Division of Cardiology, Albert Einstein College of Medicine, New York, NY 10461, USA"
}
],
"family": "Kuno",
"given": "Toshiki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Health Services Research, Faculty of Medicine, University of Tsukuba, Tsukuba 305-8575, Japan"
},
{
"name": "Graduate School of Comprehensive Human Sciences, University of Tsukuba, Tsukuba 305-8575, Japan"
}
],
"family": "Komiyama",
"given": "Jun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Health Services Research and Development Center, University of Tsukuba, Tsukuba 305-8575, Japan"
}
],
"family": "Uda",
"given": "Kazuaki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Cancer Center, Institute for Cancer Control, Tokyo 104-0045, Japan"
}
],
"family": "Miyamoto",
"given": "Yoshihisa",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-0176-2379",
"affiliation": [
{
"name": "Graduate School of Comprehensive Human Sciences, University of Tsukuba, Tsukuba 305-8575, Japan"
},
{
"name": "Health Services Research and Development Center, University of Tsukuba, Tsukuba 305-8575, Japan"
}
],
"authenticated-orcid": false,
"family": "Taniguchi",
"given": "Yuta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Health Services Research, Faculty of Medicine, University of Tsukuba, Tsukuba 305-8575, Japan"
},
{
"name": "Department of Emergency and Critical Care Medicine, Tsukuba Memorial Hospital, Tsukuba 305-8575, Japan"
}
],
"family": "Abe",
"given": "Toshikazu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Health Services Research, Faculty of Medicine, University of Tsukuba, Tsukuba 305-8575, Japan"
}
],
"family": "Ishimaru",
"given": "Miho",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4277-4509",
"affiliation": [
{
"name": "Department of Health Services Research, Faculty of Medicine, University of Tsukuba, Tsukuba 305-8575, Japan"
},
{
"name": "Health Services Research and Development Center, University of Tsukuba, Tsukuba 305-8575, Japan"
}
],
"authenticated-orcid": false,
"family": "Adomi",
"given": "Motohiko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Health Services Research, Faculty of Medicine, University of Tsukuba, Tsukuba 305-8575, Japan"
},
{
"name": "Health Services Research and Development Center, University of Tsukuba, Tsukuba 305-8575, Japan"
}
],
"family": "Tamiya",
"given": "Nanako",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Health Services Research, Faculty of Medicine, University of Tsukuba, Tsukuba 305-8575, Japan"
},
{
"name": "Health Services Research and Development Center, University of Tsukuba, Tsukuba 305-8575, Japan"
}
],
"family": "Iwagami",
"given": "Masao",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Medicine",
"container-title-short": "JCM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
27
]
],
"date-time": "2021-12-27T06:00:54Z",
"timestamp": 1640584854000
},
"deposited": {
"date-parts": [
[
2024,
7,
23
]
],
"date-time": "2024-07-23T19:01:26Z",
"timestamp": 1721761286000
},
"indexed": {
"date-parts": [
[
2025,
2,
21
]
],
"date-time": "2025-02-21T19:29:40Z",
"timestamp": 1740166180093,
"version": "3.37.3"
},
"is-referenced-by-count": 10,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
12,
26
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
26
]
],
"date-time": "2021-12-26T00:00:00Z",
"timestamp": 1640476800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2077-0383/11/1/116/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "116",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
12,
26
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
26
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1101/2020.12.28.20248950",
"doi-asserted-by": "crossref",
"key": "ref_1",
"unstructured": "Sallam, M. (2021). COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines, 9."
},
{
"DOI": "10.1016/j.ijid.2020.10.093",
"article-title": "Potential mechanisms of nafamostat therapy for severe COVID-19 pneumonia with disseminated intravascular coagulation",
"author": "Takahashi",
"doi-asserted-by": "crossref",
"first-page": "529",
"journal-title": "Int. J. Infect. Dis.",
"key": "ref_2",
"volume": "102",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-05985-9",
"article-title": "Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "586",
"journal-title": "Intensive Care Med.",
"key": "ref_3",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.3390/ijms22062815",
"doi-asserted-by": "crossref",
"key": "ref_4",
"unstructured": "Cho, T., Han, H.-S., Jeong, J., Park, E.-M., and Shim, K.-S. (2021). A Novel Computational Approach for the Discovery of Drug Delivery System Candidates for COVID-19. Int. J. Mol. Sci., 22."
},
{
"DOI": "10.3390/v12060629",
"doi-asserted-by": "crossref",
"key": "ref_5",
"unstructured": "Yamamoto, M., Kiso, M., Sakai-Tagawa, Y., Iwatsuki-Horimoto, K., Imai, M., Takeda, M., Kinoshita, N., Ohmagari, N., Gohda, J., and Semba, K. (2020). The Anticoagulant Nafamostat Potently Inhibits SARS-CoV-2 S Protein-Mediated Fusion in a Cell Fusion Assay System and Viral Infection In Vitro in a Cell-Type-Dependent Manner. Viruses, 12."
},
{
"DOI": "10.1016/j.jiac.2020.08.001",
"article-title": "Clinical improvement in a patient with severe coronavirus disease 2019 after administration of hydroxychloroquine and continuous hemodiafiltlation with nafamostat mesylate",
"author": "Iwasaka",
"doi-asserted-by": "crossref",
"first-page": "1319",
"journal-title": "J. Infect. Chemother.",
"key": "ref_6",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1186/s13054-020-03078-z",
"article-title": "Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with COVID-19: A case series",
"author": "Doi",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Crit. Care",
"key": "ref_7",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.05.072",
"article-title": "Three cases of treatment with nafamostat in elderly patients with COVID-19 pneumonia who need oxygen therapy",
"author": "Jang",
"doi-asserted-by": "crossref",
"first-page": "500",
"journal-title": "Int. J. Infect. Dis.",
"key": "ref_8",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2021.101169",
"article-title": "Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: A randomised Phase II clinical trial",
"author": "Zhuravel",
"doi-asserted-by": "crossref",
"first-page": "101169",
"journal-title": "EClinicalMedicine",
"key": "ref_9",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1016/j.je.2016.09.009",
"article-title": "Validity of diagnoses, procedures, and laboratory data in Japanese administrative data",
"author": "Yamana",
"doi-asserted-by": "crossref",
"first-page": "476",
"journal-title": "J. Epidemiology",
"key": "ref_10",
"volume": "27",
"year": "2017"
},
{
"DOI": "10.1093/aje/kwm324",
"article-title": "Immortal time bias in pharmaco-epidemiology",
"author": "Suissa",
"doi-asserted-by": "crossref",
"first-page": "492",
"journal-title": "Am. J. Epidemiol.",
"key": "ref_11",
"volume": "167",
"year": "2008"
},
{
"DOI": "10.1093/aje/kwq433",
"article-title": "Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries",
"author": "Quan",
"doi-asserted-by": "crossref",
"first-page": "676",
"journal-title": "Am. J. Epidemiol.",
"key": "ref_12",
"volume": "173",
"year": "2011"
},
{
"DOI": "10.2176/nmc.47.291",
"article-title": "Indications for Computed Tomography in Patients With Mild Head Injury",
"author": "Ono",
"doi-asserted-by": "crossref",
"first-page": "291",
"journal-title": "Neurol. Med. -Chir.",
"key": "ref_13",
"volume": "47",
"year": "2007"
},
{
"DOI": "10.1002/sim.4780100410",
"article-title": "Multiple imputation in health-care databases: An overview and some applications",
"author": "Rubin",
"doi-asserted-by": "crossref",
"first-page": "585",
"journal-title": "Stat. Med.",
"key": "ref_14",
"volume": "10",
"year": "1991"
},
{
"DOI": "10.1177/1536867X1401400410",
"article-title": "Analysis of Partially Observed Clustered Data using Generalized Estimating Equations and Multiple Imputation",
"author": "Aloisio",
"doi-asserted-by": "crossref",
"first-page": "863",
"journal-title": "Stata Journal: Promot. Commun. Stat. Stata",
"key": "ref_15",
"volume": "14",
"year": "2014"
},
{
"DOI": "10.1093/aje/kwj149",
"article-title": "Variable Selection for Propensity Score Models",
"author": "Brookhart",
"doi-asserted-by": "crossref",
"first-page": "1149",
"journal-title": "Am. J. Epidemiol.",
"key": "ref_16",
"volume": "163",
"year": "2006"
},
{
"DOI": "10.1080/00031305.1985.10479383",
"article-title": "Constructing a Control Group Using Multivariate Matched Sampling Methods That Incorporate the Propensity Score",
"author": "Rosenbaum",
"doi-asserted-by": "crossref",
"first-page": "33",
"journal-title": "Am. Stat.",
"key": "ref_17",
"volume": "39",
"year": "1985"
},
{
"DOI": "10.1080/00273171.2011.568786",
"article-title": "An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies",
"author": "Austin",
"doi-asserted-by": "crossref",
"first-page": "399",
"journal-title": "Multivar. Behav. Res.",
"key": "ref_18",
"volume": "46",
"year": "2011"
},
{
"DOI": "10.1002/sim.3697",
"article-title": "Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples",
"author": "Austin",
"doi-asserted-by": "crossref",
"first-page": "3083",
"journal-title": "Stat. Med.",
"key": "ref_19",
"volume": "28",
"year": "2009"
},
{
"DOI": "10.1016/j.ajo.2009.08.024",
"article-title": "Propensity score methods",
"author": "Rubin",
"doi-asserted-by": "crossref",
"first-page": "7",
"journal-title": "Am. J. Ophthalmol.",
"key": "ref_20",
"volume": "149",
"year": "2010"
},
{
"DOI": "10.1186/s40560-018-0308-6",
"article-title": "The Japanese Clinical Practice Guideline for acute kidney injury 2016",
"author": "Doi",
"doi-asserted-by": "crossref",
"first-page": "48",
"journal-title": "J. Intensiv. Care",
"key": "ref_21",
"volume": "6",
"year": "2018"
},
{
"DOI": "10.3389/fphar.2021.596855",
"article-title": "Therapeutic Potentials of Antiviral Plants Used in Traditional African Medicine With COVID-19 in Focus: A Nigerian Perspective",
"author": "Attah",
"doi-asserted-by": "crossref",
"first-page": "596855",
"journal-title": "Front. Pharmacol.",
"key": "ref_22",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.amsu.2021.102560",
"article-title": "Clinical efficacy of Nafamostat Mesylate in combination with Favipiravir for COVID-19 pneumonia treatment review article",
"author": "Abduljabbar",
"doi-asserted-by": "crossref",
"first-page": "102560",
"journal-title": "Ann. Med. Surg.",
"key": "ref_23",
"volume": "68",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n1703",
"doi-asserted-by": "crossref",
"key": "ref_24",
"unstructured": "WHO (2021). Update to living WHO guideline on drugs for COVID-19. BMJ, 374, n1703. Available online: https://www.bmj.com/content/374/bmj.n1703.long."
},
{
"DOI": "10.1128/AAC.00754-20",
"article-title": "Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "e00754-20",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_25",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1470",
"article-title": "Clinical epidemiology of hospitalized patients with COVID-19 in Japan: Report of the COVID-19 REGISTRY JAPAN",
"author": "Matsunaga",
"doi-asserted-by": "crossref",
"first-page": "e3677",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_26",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1198",
"article-title": "COVID-19: Risk factors for severe disease and death",
"author": "Jordan",
"doi-asserted-by": "crossref",
"first-page": "m1198",
"journal-title": "BMJ",
"key": "ref_27",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1012",
"article-title": "Risk Factors for Intensive Care Unit Admission and In-hospital Mortality Among Hospitalized Adults Identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET)",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "e206",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_28",
"volume": "72",
"year": "2020"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2077-0383/11/1/116"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Association between Nafamostat Mesylate and In-Hospital Mortality in Patients with Coronavirus Disease 2019: A Multicenter Observational Study",
"type": "journal-article",
"volume": "11"
}
inokuchi2