Feb 21 |
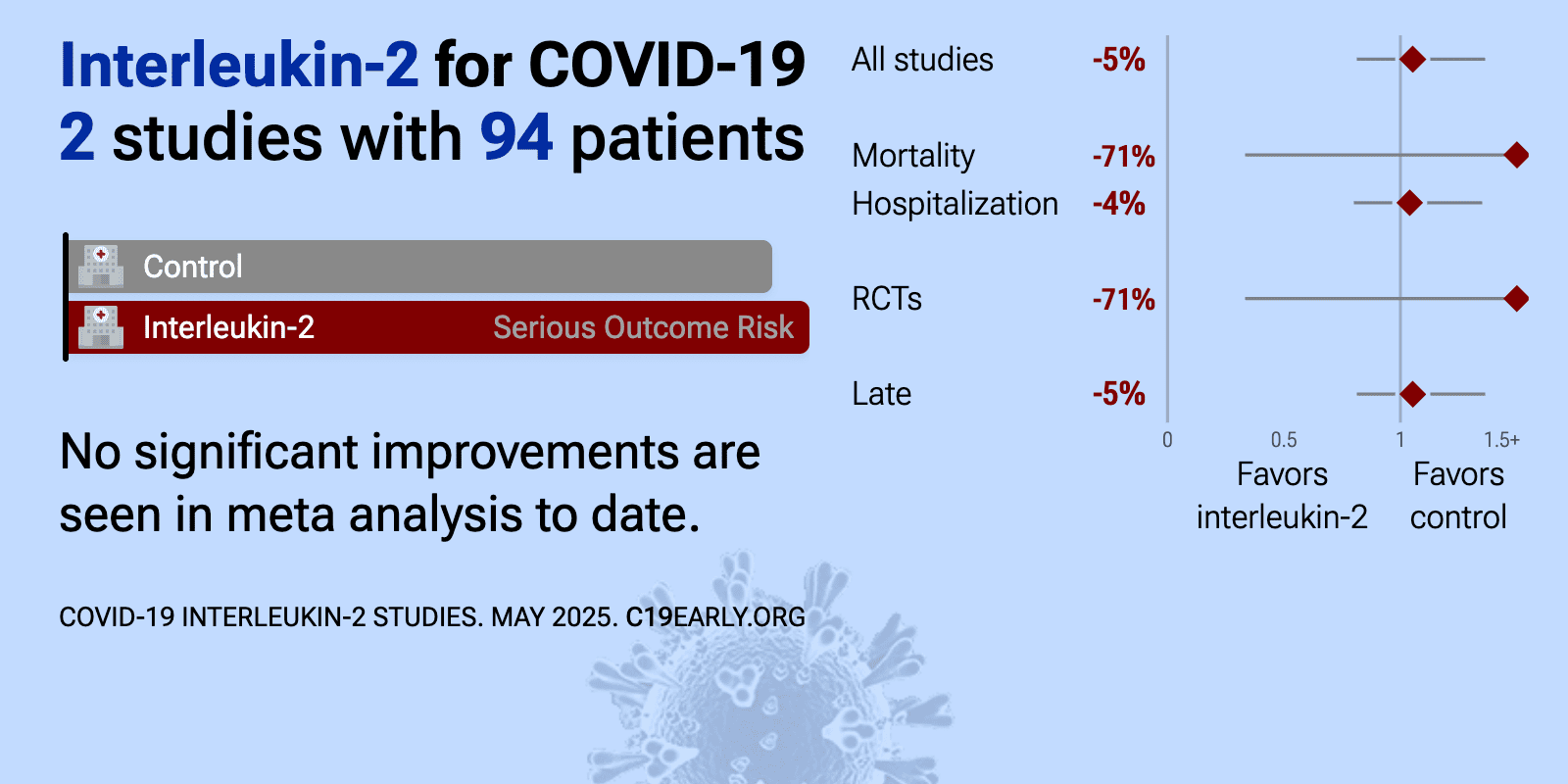
Meta-analysis of interleukin-2 studies | |
| Meta-analysis of interleukin-2 studies | ||
Apr 28 2022 |
et al., Revista da Sociedade Brasileira de Medicina Tropical, doi:10.1590/0037-8682-0565-2022 (date from preprint) | Efficacy and safety of Ixekizumab vs. low-dose IL-2 vs. Colchicine vs. standard of care in the treatment of patients hospitalized with moderate-to-critical COVID-19: A pilot randomized clinical trial (STRUCK: Survival Trial Using Cytokine Inhibitors) |
| 71% higher mortality (p=0.64) and 90% worse improvement (p=0.42). Open label RCT late stage hospitalized patients in Brazil showing no significant difference with IL-2 or ixekizumab treatment. Mortality was lower and recovery was greater with colchicine, without statistical significance. | ||
Jan 18 2021 |
et al., Experimental and Therapeutic Medicine, doi:10.3892/etm.2021.9658 | Recombinant interleukin-2 stimulates lymphocyte recovery in patients with severe COVID-19 |
| 4% longer hospitalization (p=0.78). PSM retrospective 59 hospitalized patients with severe COVID-19 showing that recombinant human interleukin-2 (rIL-2) treatment significantly increased lymphocyte counts. There were no significant differences in C-reactive protein or IL-6 .. | ||