Sep 20 2022 |
et al., NCT04439006 | Randomized Double-Blind Phase 2 Trial of Ibrutinib Versus Standard Treatment for COVID-19 Illness Requiring Hospitalization With Safety Lead-In |
| 10 patient ibrutinib late treatment RCT with results not reported over 3 years after completion. | ||
Mar 24 2022 |
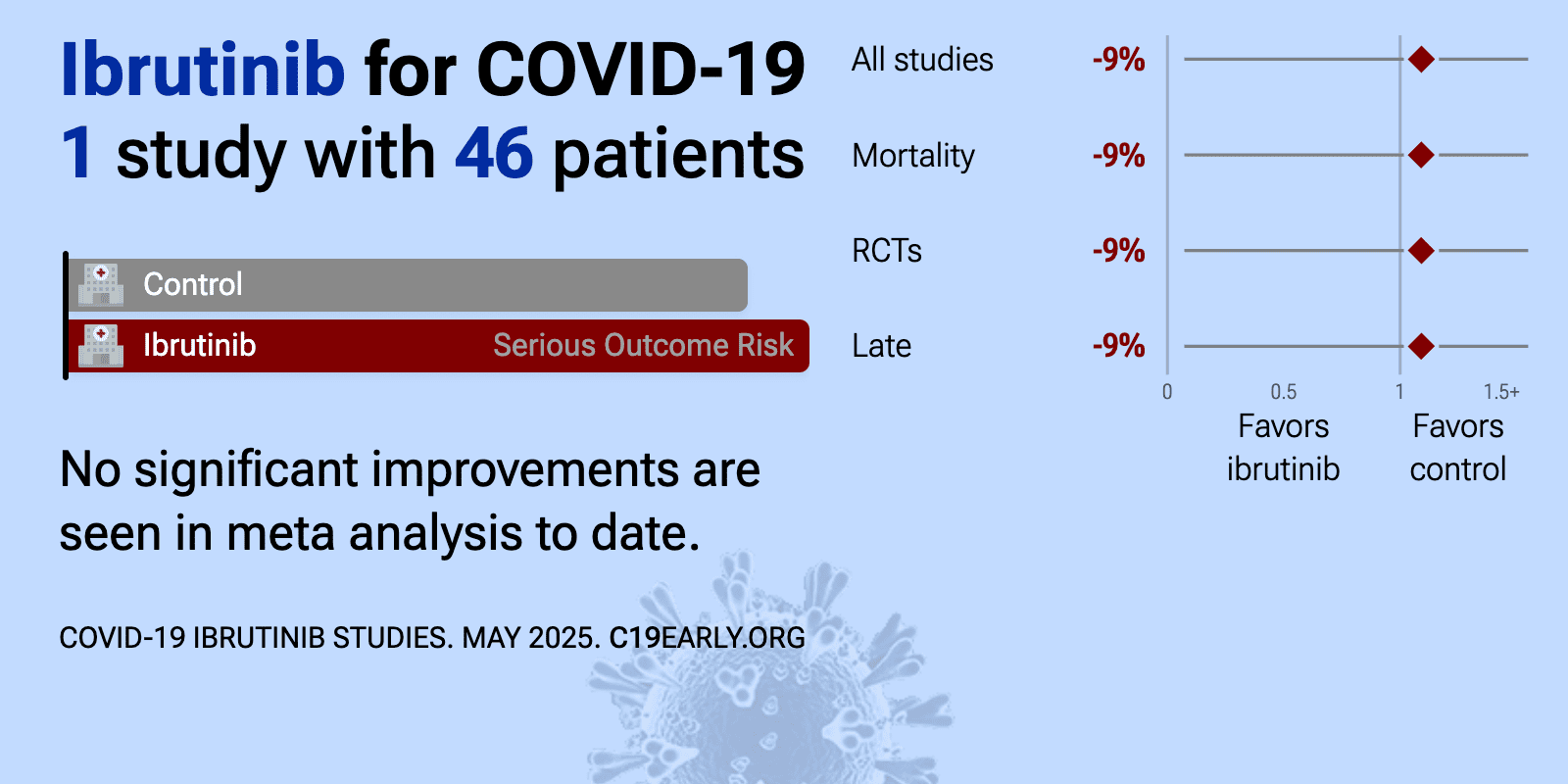
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofac104 | Ibrutinib for Hospitalized Adults With Severe Coronavirus Disease 2019 Infection: Results of the Randomized, Double-Blind, Placebo-Controlled iNSPIRE Study |
| 9% higher mortality (p=1), 9% lower hospital discharge (p=1), and 35% lower progression (p=0.7). RCT 46 hospitalized patients with severe COVID-19 showing no significant differences with ibrutinib treatment. | ||