Effects of COVID-19 Convalescent Plasma (CCP) on Coronavirus-associated Complications in Hospitalized Patients (CAPRI)
et al., NCT04421404, CAPRI, NCT04421404, Aug 2021
RCT 34 hospitalized patients in the USA, showing no significant difference with convalescent plasma treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 212.5% higher, RR 3.12, p = 0.47, treatment 1 of 16 (6.2%), control 0 of 18 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 28.
|
|
risk of death, 12.5% higher, RR 1.12, p = 1.00, treatment 1 of 16 (6.2%), control 1 of 18 (5.6%), all cause, day 28.
|
|
risk of mechanical ventilation, 425.0% higher, RR 5.25, p = 0.21, treatment 2 of 16 (12.5%), control 0 of 18 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 28.
|
|
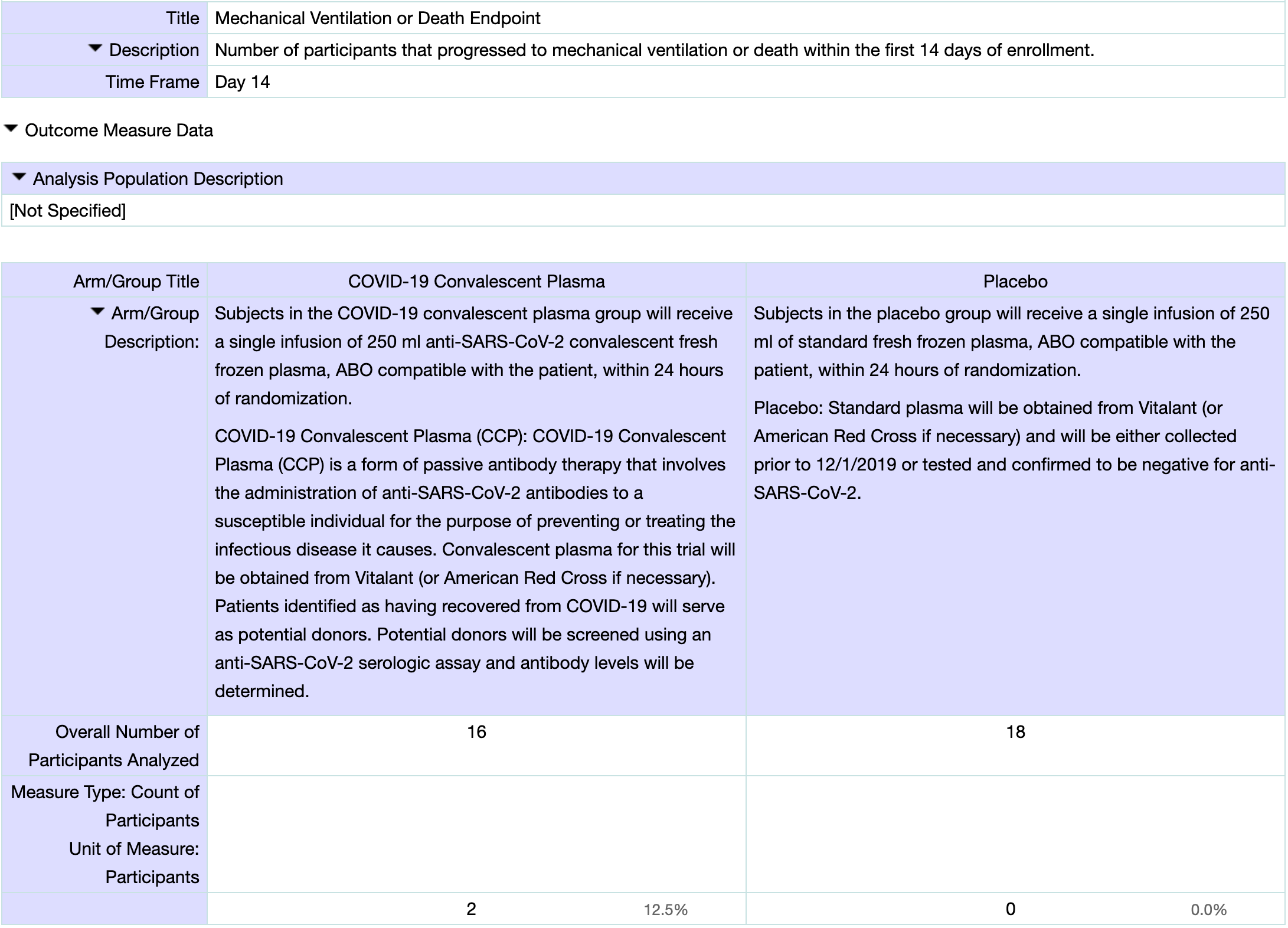
risk of progression, 425.0% higher, RR 5.25, p = 0.21, treatment 2 of 16 (12.5%), control 0 of 18 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), death or mechanical ventilation, day 28, primary outcome.
|
|
risk of progression, 425.0% higher, RR 5.25, p = 0.21, treatment 2 of 16 (12.5%), control 0 of 18 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), death or mechanical ventilation, day 14, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hsue et al., 23 Aug 2021, Double Blind Randomized Controlled Trial, placebo-controlled, USA, preprint, 1 author, study period 9 June, 2020 - 30 April, 2021, trial NCT04421404 (history) (CAPRI).