FRAGILE-COLCOVID19: A Clinical Trial Based on Early Administration of an Oral Combination of Colchicine and Prednisone in Elderly Patients with COVID-19 in Geriatric Facilities
et al., Clinical Drug Investigation, doi:10.1007/s40261-022-01201-2, FRAGILE-COLCOVID19, NCT04492358, Sep 2022
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
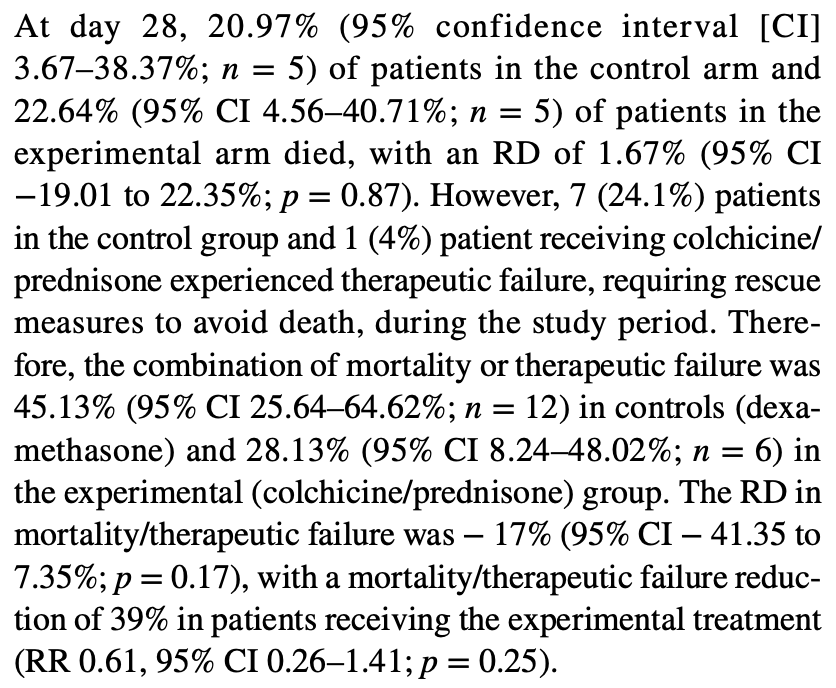
RCT 54 elderly patients in Spain comparing colchicine + prednisone vs. dexamethasone, showing lower combined mortality/therapeutic failure with colchicine + prednisone, but no significant difference in mortality. Data appears unreliable, for example authors report 5 deaths (20.97%) in the control arm, however 20.97% does not match the reported (or any) number of patients. Similarly for combined mortality/therapeutic failure where authors report 12 (45.13%) and 6 (28.13%).
|
risk of death, 16.0% higher, RR 1.16, p = 1.00, treatment 5 of 25 (20.0%), control 5 of 29 (17.2%).
|
|
mortality/therapeutic failure, 42.0% lower, RR 0.58, p = 0.25, treatment 6 of 25 (24.0%), control 12 of 29 (41.4%), NNT 5.8, combined mortality/therapeutic failure.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hernández-Rodríguez et al., 29 Sep 2022, Randomized Controlled Trial, Spain, peer-reviewed, 90 authors, study period March 2020 - October 2020, this trial compares with another treatment - results may be better when compared to placebo, this trial uses multiple treatments in the treatment arm (combined with prednisone) - results of individual treatments may vary, trial NCT04492358 (history) (FRAGILE-COLCOVID19).
Contact: jhernan@clinic.cat.
FRAGILE-COLCOVID19: A Clinical Trial Based on Early Administration of an Oral Combination of Colchicine and Prednisone in Elderly Patients with COVID-19 in Geriatric Facilities
Clinical Drug Investigation, doi:10.1007/s40261-022-01201-2
Background Unprotected and fragile elderly people in nursing homes experienced the highest mortality rates during the initial coronavirus disease 2019 (COVID-19) pandemic. Objective Our aim was to study the role of two oral anti-inflammatory drugs, colchicine and prednisone, in elderly patients with COVID-19 in geriatric centers. Methods A phase II/III, randomized, controlled, multicenter clinical trial was performed in a geriatric population comparing the efficacy and safety of an oral combination of prednisone (60 mg/day for 3 days) and colchicine (at loading doses of 1-1.5 mg/day for 3 days, followed by 0.5 mg/day for 11 days) with the standard treatment, based on intravenous dexamethasone. Primary endpoints assessed the efficacy in reducing death or the modified endpoint death/therapeutic failure to the study drugs over a 28-day period, while secondary endpoints included safety, laboratory changes, and additional therapies used. Results Fifty-four patients (35 female/19 male) were enrolled, 25 (46.3%) of whom were allocated to the experimental arm and 29 (53.7%) to the control arm. At day 28, no differences in deaths were observed. The combination of mortality or therapeutic failure occurred in 12 (45.13%) patients receiving dexamethasone and 6 (28.13%) patients receiving colchicine/ prednisone, resulting in a reduction of risk difference (RD) of − 17% (p = 0.17), with an average reduction of 39% (risk ratio [RR] 0.61) in patients receiving colchicine/prednisone (p = 0.25). Control patients received higher amounts of additional glucocorticoids (p = 0.0095) over a longer time frame (p = 0.0003). Colchicine/prednisone significantly reduced ferritin levels at day 14, as well as d-dimer and lactate dehydrogenase (LDH) levels at day 28. Adverse events were similar in both groups. Conclusions The combination colchicine/prednisone compared with intravenous dexamethasone has shown a remarkable trend to increase disease survival over a 28-day period in elderly patients requiring oxygen therapy in geriatric centers, without safety issues.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1007/ s40261-022-01201-2.
Declarations Conflict of interest José Hernández-Rodríguez, Julio Durán-Sanclemente, Sergio Prieto-González, Olga Araújo, Teresa Hospital-Vidal, Georgina Casanovas, Víctor Sapena, José Luis Blanco, and Alfonso López-Soto declare no conflicts of interest in this work.
Ethics approval This study was approved by the Clinical Research Ethics Committee at the Hospital Clínic of Barcelona (EudraCT Number 2020-002462-14).
Consent to participate All patients or legally authorized representatives signed the informed consent form.
Consent for publication Not applicable. Author contributions JHR and JLB were involved in the conceptualization and design of the study protocol. ALS and JDS allowed the launch of the study by providing the geriatric facilities and encouraging the participation of their healthcare personnel. JDS, SPG, OA, THV, and all Members of the FRAGILE-COLCOVID19 Study Group recruited patients, collected data, and/or monitored patients enrolled in the study. VS and GC performed the statistical analysis and summarized the statistical data for the manuscript. JHR was responsible for funding acquisition, project administration and data analysis, and wrote the manuscript. All authors contributed to the critical revision and editing of the manuscript and approved its final version. Open Access This article is licensed under a..
References
Abdeen, Abu-Fanne, Bdeir, Divergent impacts of tocilizumab and colchicine in COVID-19-associated coagulopathy: the role of alpha-defensins, Br J Haematol
Absalon-Aguilar, Rull-Gabayet, Perez-Fragoso, Colchicine is safe though ineffective in the treatment of severe COVID-19: a randomized clinical trial (COLCHIVID), J Gen Intern Med
Alsultan, Obeid, Alsamarrai, Efficacy of colchicine and budesonide in improvement outcomes of patients with coronavirus infection 2019 in Damascus, Syria: a randomized control trial, Interdiscip Perspect Infect Dis
Angelidis, Kotsialou, Kossyvakis, Colchicine pharmacokinetics and mechanism of action, Curr Pharm Des
Barnes, Anti-inflammatory actions of glucocorticoids: molecular mechanisms, Clin Sci (Lond)
Brunetti, Diawara, Tsai, Colchicine to weather the cytokine storm in hospitalized patients with COVID-19, J Clin Med
Chen, Dai, Mo, Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a single-centered, retrospective study, J Gerontol A Biol Sci Med Sci
Chiu, Lo, Shen, Colchicine use in patients with COVID-19: a systematic review and meta-analysis, PLoS ONE
Cisco, Afonso, Aibar, Alemany, Aparicio et al., COVID-19 Dashboard by the Center for Systems Science and Engineering
Da, Roura-Piloto, Moral-Escudero, Colchicine in recently hospitalized patients with COVID-19: a randomized controlled trial (COL-COVID), Int J Gen Med
De-Miguel-Balsa, Estevan-Ortega, Sempere-Selva, Can we still consider treatment with colchicine effective in SARS-COV-2 infection? Systematic review, meta-analysis, and trial sequential analysis, Eur Rev Med Pharmacol Sci
Deftereos, Giannopoulos, Vrachatis, Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial, JAMA Netw Open
Della-Torre, Della-Torre, Kusanovic, Treating COVID-19 with colchicine in community healthcare setting, Clin Immunol
Diaz, Orlandini, Castellana, Effect of colchicine vs usual care alone on intubation and 28-day mortality in patients hospitalized with COVID-19: a randomized clinical trial, JAMA Netw Open
Dorward, Yu, Hayward, Colchicine for COVID-19 in adults in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial, Br J Gen Pract
Drosos, Pelechas, Drossou, Voulgari, Colchicine against SARS-CoV-2 infection: what is the evidence?, Rheumatol Ther
Elshafei, El-Bardissy, Khalil, Colchicine use might be associated with lower mortality in COVID-19 patients: a metaanalysis, Eur J Clin Invest
Ferron, Rochdi, Jusko, Scherrmann, Oral absorption characteristics and pharmacokinetics of colchicine in healthy volunteers after single and multiple doses, J Clin Pharmacol
Gaitan-Duarte, Alvarez-Moreno, Rincon-Rodriguez, Effectiveness of rosuvastatin plus colchicine, emtricitabine/ tenofovir and combinations thereof in hospitalized patients with COVID-19: a pragmatic, open-label randomized trial, EClinical-Medicine
Golpour, Mousavi, Alimohammadi, The effectiveness of colchicine as an anti-inflammatory drug in the treatment of coronavirus disease 2019: meta-analysis, Int J Immunopathol Pharmacol
González-Gay, Mayo, Castañeda, Cifrián, Hernández-Rodríguez, Tocilizumab: from the rheumatology practice to the fight against COVID-19, a virus infection with multiple faces, Expert Opin Biol Ther
Group, Horby, Lim, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med
Hariyanto, Halim, Jodhinata, Yanto, Kurniawan, Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis, Clin Exp Pharmacol Physiol
Karakas, Erden, Guven, Reducing length of hospital stay with colchicine, J Infect Dev Ctries
Kow, Lee, Ramachandram, Hasan, Ming et al., The effect of colchicine on mortality outcome and duration of hospital stay in patients with COVID-19: A meta-analysis of randomized trials, Immun Inflamm Dis
Lien, Lee, Weng, Repurposing colchicine in treating patients with COVID-19: a systematic review and metaanalysis, Life
Lopes, Bonjorno, Giannini, Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial, RMD Open
Lu, Yang, Liu, Inhibition of respiratory syncytial virus replication and suppression of RSV-induced airway inflammation in neonatal rats by colchicine, Biotech
Manenti, Maggiore, Fiaccadori, Reduced mortality in COVID-19 patients treated with colchicine: results from a retrospective, observational study, PLoS ONE
Mareev, Orlova, Plisyk, Proactive anti-inflammatory therapy with colchicine in the treatment of advanced stages of new coronavirus infection. The first results of the COL-ORIT study, Kardiologiia
Mehta, Cron, Hartwell, Manson, Tattersall, Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome, Lancet Rheumatol
Mehta, Mcauley, Brown, COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet
Mehta, Patel, Chavda, Patel, Efficacy and safety of colchicine in COVID-19: a meta-analysis of randomised controlled trials, RMD Open
Mikolajewska, Fischer, Piechotta, Colchicine for the treatment of COVID-19, Cochrane Database Syst Rev
Nawangsih, Kusmala, Rakhmat, Colchicine and mortality in patients with coronavirus disease 2019 (COVID-19) pneumonia: a systematic review, meta-analysis, and meta-regression, Int Immunopharmacol
Piantoni, Patroni, Toniati, Why not to use colchicine in COVID-19? An oldanti-inflammatory drug for a novel auto-inflammatory disease, Rheumatology
Pickup, Clinical pharmacokinetics of prednisone and prednisolone, Clin Pharmacokinet
Pinzon, Arango, Betancur, Clinical outcome of patients with COVID-19 Pneumonia treated with corticosteroids and colchicine in Colombia, Ann Clin Microbiol Antimicrob
Pinzon, Ortiz, Holguin, Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia, PLoS ONE
Pourdowlat, Saghafi, Mozafari, Efficacy and safety of colchicine treatment in patients with COVID-19: a prospective, multicenter, randomized clinical trial, Phytother Res
Ranjbar, Moghadami, Mirahmadizadeh, Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial, BMC Infect Dis
Research, Covid, Welch, Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: results of an international multi-centre study, Age Ageing
Richardson, Hirsch, Narasimhan, Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area, JAMA
Ronchetti, Ayroldi, Ricci, Gentili, Migliorati et al., A glance at the use of glucocorticoids in rare inflammatory and autoimmune diseases: Still an indispensable pharmacological tool?, Front Immunol
Rubio, Del Castillo, De La Hera, Arrabal, Ruiz et al., Effectiveness of corticoid pulses in patients with cytokine storm syndrome induced by SARS-CoV-2 infection, Med Clin (Barc)
Ruiz-Irastorza, Pijoan, Bereciartua, Second week methyl-prednisolone pulses improve prognosis in patients with severe coronavirus disease 2019 pneumonia: an observational comparative study using routine care data, PLoS ONE
Salinas, Andino, Palma, Valencia, Figueroa et al., Early use of corticosteroids in non-critical patients with COVID-19 pneumonia (PREDCOVID): a structured summary of a study protocol for a randomised controlled trial, Trials
Sandhu, Tieng, Chilimuri, Franchin, A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe COVID-19 infection, Can J Infect Dis Med Microbiol
Sanghavi, Bansal, Kaur, Impact of colchicine on mortality and morbidity in COVID-19: a systematic review, Ann Med
Sarwar, Ali, Fatima, Sarfraz, Sarfraz et al., Colchicine, COVID-19 and hematological parameters: a metaanalysis, J Clin Lab Anal
Scarsi, Piantoni, Colombo, Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome, Ann Rheum Dis
Soriano, Soriano, Espinosa, Current therapeutic options for the main monogenic autoinflammatory diseases and PFAPA syndrome: Evidence-based approach and proposal of a practical guide, Front Immunol
Sterne, Murthy, Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a metaanalysis, JAMA
Tardif, Bouabdallaoui, Allier, Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial, Lancet Respir Med
Wang, Zhu, Dai, Specific interleukin-1 inhibitors, specific interleukin-6 inhibitors, and GM-CSF blockades for COVID-19 (at the edge of sepsis): a systematic review, Front Pharmacol
Zein, Raffaello, Effect of colchicine on mortality in patients with COVID-19-a systematic review and meta-analysis, Diabetes Metab Syndr
Zeng, Deng, Cui, A predictive model for the severity of COVID-19 in elderly patients, Aging
Zhang, Shang, Fan, The efficacy and safety of janus kinase inhibitors for patients with COVID-19: a living systematic review and meta-analysis, Front Med
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1007/s40261-022-01201-2",
"ISSN": [
"1173-2563",
"1179-1918"
],
"URL": "http://dx.doi.org/10.1007/s40261-022-01201-2",
"alternative-id": [
"1201"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "5 September 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 2,
"value": "29 September 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Funding",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This study was sponsored by SEID S.A. and the Hospital Clínic of Barcelona Sponsorship Program, and partially by Alchemlife Iberia S.L. SEID S.A. (Lliçà de Vall, Barcelona, Spain) also provided the Colchicina SEID<sup>®</sup> tablets used in the study. None of the sponsors had any role in the study design or development, data analysis, or manuscript writing."
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "José Hernández-Rodríguez, Julio Durán-Sanclemente, Sergio Prieto-González, Olga Araújo, Teresa Hospital-Vidal, Georgina Casanovas, Víctor Sapena, José Luis Blanco, and Alfonso López-Soto declare no conflicts of interest in this work."
},
{
"group": {
"label": "Ethics approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "This study was approved by the Clinical Research Ethics Committee at the Hospital Clínic of Barcelona (EudraCT Number 2020-002462-14)."
},
{
"group": {
"label": "Consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 5,
"value": "All patients or legally authorized representatives signed the informed consent form."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 6,
"value": "Not applicable."
},
{
"group": {
"label": "Availability of data and material",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 7,
"value": "The data generated during the current study are available from the corresponding author on reasonable request."
},
{
"group": {
"label": "Code availability",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 8,
"value": "Not applicable."
},
{
"group": {
"label": "Author contributions",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 9,
"value": "JHR and JLB were involved in the conceptualization and design of the study protocol. ALS and JDS allowed the launch of the study by providing the geriatric facilities and encouraging the participation of their healthcare personnel. JDS, SPG, OA, THV, and all Members of the FRAGILE-COLCOVID19 Study Group recruited patients, collected data, and/or monitored patients enrolled in the study. VS and GC performed the statistical analysis and summarized the statistical data for the manuscript. JHR was responsible for funding acquisition, project administration and data analysis, and wrote the manuscript. All authors contributed to the critical revision and editing of the manuscript and approved its final version."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2357-2015",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hernández-Rodríguez",
"given": "José",
"sequence": "first"
},
{
"affiliation": [],
"family": "Durán-Sanclemente",
"given": "Julio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7242-5265",
"affiliation": [],
"authenticated-orcid": false,
"family": "Prieto-González",
"given": "Sergio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1763-1880",
"affiliation": [],
"authenticated-orcid": false,
"family": "Araújo",
"given": "Olga",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hospital-Vidal",
"given": "Teresa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1629-5475",
"affiliation": [],
"authenticated-orcid": false,
"family": "Casanovas",
"given": "Georgina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4379-6486",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sapena",
"given": "Víctor",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2010-2744",
"affiliation": [],
"authenticated-orcid": false,
"family": "Blanco",
"given": "José Luis",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4297-3217",
"affiliation": [],
"authenticated-orcid": false,
"family": "López-Soto",
"given": "Alfonso",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Afonso",
"given": "Francisco J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aibar",
"given": "Jesús",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alemany",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aparicio",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asensio",
"given": "Yolanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aldea-Parés",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Azuaga",
"given": "Ana B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barilaro",
"given": "Giuseppe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benages",
"given": "Nieves",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cajiao",
"given": "Katherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Calvo",
"given": "Júlia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cañueto",
"given": "Maria del Carme",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Capdevila-Reniu",
"given": "Aina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carbonell",
"given": "Irene",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Costafreda",
"given": "Miriam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cuzco",
"given": "Cecilia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de-Daniel-Bisbe",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Doménech",
"given": "Gema",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Doménech",
"given": "Mónica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Espinosa",
"given": "Gerard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Feliu",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Foncillas",
"given": "Alberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gabara",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gámez",
"given": "Gloria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García-Gutiérrez",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García-Jarque",
"given": "Lucía",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García-Ortega",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gómez",
"given": "Begoña",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gómez-Caverzaschi",
"given": "Verónica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gómez-Urbano",
"given": "Vanesa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gonzalo",
"given": "Tania",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grafia",
"given": "Ignacio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guasch",
"given": "Neus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guillén",
"given": "Mar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guió",
"given": "Ana M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Illa",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Inzitari",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Joyera",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ladino",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luzko-Scheid",
"given": "Irina S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lledó",
"given": "Gema M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "López-Guerra",
"given": "Néstor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marco",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Masdeu",
"given": "Guillem",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matas-García",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Macaya",
"given": "Irene",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Masanés",
"given": "Ferran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mayor",
"given": "Miriam I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Milisenda",
"given": "José C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montero",
"given": "Montse",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montes",
"given": "Verónica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montoya-Rodés",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moragas",
"given": "Núria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morancho",
"given": "Alma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moreno",
"given": "Pedro J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Naval",
"given": "José",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ortega",
"given": "Josep",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pahisa",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pellicé",
"given": "Martina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pilarcikova",
"given": "Sona",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pujol",
"given": "Ester",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rivero",
"given": "Elisabet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ribot",
"given": "Joan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ríos-Garcés",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodríguez-Núñez",
"given": "Olga",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saavedra",
"given": "Omar M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sacanella",
"given": "Emilio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salazar",
"given": "Adelaido",
"sequence": "additional"
},
{
"affiliation": [],
"family": "San Miguel",
"given": "Adriana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sánchez-Buitrago",
"given": "Amparo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sánchez-Palacios",
"given": "Víctor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sanz",
"given": "Ángeles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seguí",
"given": "Ferran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tomé-Pérez",
"given": "Adrià",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Torres-Elorza",
"given": "Manuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tuset",
"given": "Montse",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ventosa",
"given": "Helena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ventura",
"given": "Roser",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Viñas-Esmel",
"given": "Esther",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zamora",
"given": "Carles",
"sequence": "additional"
},
{
"affiliation": [],
"name": "FRAGILE-COLCOVID19 Study Group",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04492358",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "Clinical Drug Investigation",
"container-title-short": "Clin Drug Investig",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
9,
29
]
],
"date-time": "2022-09-29T11:02:38Z",
"timestamp": 1664449358000
},
"deposited": {
"date-parts": [
[
2022,
9,
29
]
],
"date-time": "2022-09-29T11:20:11Z",
"timestamp": 1664450411000
},
"indexed": {
"date-parts": [
[
2022,
9,
30
]
],
"date-time": "2022-09-30T04:55:48Z",
"timestamp": 1664513748369
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
9,
29
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
29
]
],
"date-time": "2022-09-29T00:00:00Z",
"timestamp": 1664409600000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
29
]
],
"date-time": "2022-09-29T00:00:00Z",
"timestamp": 1664409600000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40261-022-01201-2.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s40261-022-01201-2/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40261-022-01201-2.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2022,
9,
29
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
29
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "1201_CR1",
"unstructured": "COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2020. Available at : https://www.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6. Accessed 1 June 2022."
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "F Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet",
"key": "1201_CR2",
"unstructured": "Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1093/ageing/afab026",
"author": "C Welch",
"doi-asserted-by": "publisher",
"first-page": "617",
"issue": "3",
"journal-title": "Age Ageing",
"key": "1201_CR3",
"unstructured": "Geriatric Medicine Research C, Covid C, Welch C. Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: results of an international multi-centre study. Age Ageing. 2021;50(3):617–30.",
"volume": "50",
"year": "2021"
},
{
"DOI": "10.18632/aging.103980",
"author": "F Zeng",
"doi-asserted-by": "publisher",
"first-page": "20982",
"issue": "21",
"journal-title": "Aging (Albany NY).",
"key": "1201_CR4",
"unstructured": "Zeng F, Deng G, Cui Y, et al. A predictive model for the severity of COVID-19 in elderly patients. Aging (Albany NY). 2020;12(21):20982–96.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6775",
"author": "S Richardson",
"doi-asserted-by": "publisher",
"first-page": "2052",
"issue": "20",
"journal-title": "JAMA",
"key": "1201_CR5",
"unstructured": "Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–9.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1093/gerona/glaa089",
"author": "T Chen",
"doi-asserted-by": "publisher",
"first-page": "1788",
"issue": "9",
"journal-title": "J Gerontol A Biol Sci Med Sci",
"key": "1201_CR6",
"unstructured": "Chen T, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020;75(9):1788–95.",
"volume": "75",
"year": "2020"
},
{
"key": "1201_CR7",
"unstructured": "Infographic: COVID-19 in care homes—European Centre for Disease Prevention and Control. 2020. Available at: https://www.ecdceuropa.eu/en/publications-data/covid-19-care-homes-infographic. Accessed 11 Apr 2022."
},
{
"key": "1201_CR8",
"unstructured": "Data on elderly people living in nursing homes or alone in the EU. 2020. Available at: https://www.europarleuropa.eu/doceo/document/E-9-2020-006697_ENhtml. Accessed 11 Apr 2022."
},
{
"DOI": "10.1080/14712598.2020.1770222",
"author": "MA González-Gay",
"doi-asserted-by": "publisher",
"first-page": "717",
"issue": "7",
"journal-title": "Expert Opin Biol Ther",
"key": "1201_CR9",
"unstructured": "González-Gay MA, Mayo J, Castañeda S, Cifrián JM, Hernández-Rodríguez J. Tocilizumab: from the rheumatology practice to the fight against COVID-19, a virus infection with multiple faces. Expert Opin Biol Ther. 2020;20(7):717–23.",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(20)30096-5",
"author": "P Mehta",
"doi-asserted-by": "publisher",
"first-page": "e358",
"issue": "6",
"journal-title": "Lancet Rheumatol.",
"key": "1201_CR10",
"unstructured": "Mehta P, Cron RQ, Hartwell J, Manson JJ, Tattersall RS. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. 2020;2(6):e358–67.",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"author": "P Mehta",
"doi-asserted-by": "publisher",
"first-page": "1033",
"issue": "10229",
"journal-title": "Lancet",
"key": "1201_CR11",
"unstructured": "Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.medcli.2020.04.018",
"author": "JL Callejas Rubio",
"doi-asserted-by": "publisher",
"first-page": "159",
"issue": "4",
"journal-title": "Med Clin (Barc)",
"key": "1201_CR12",
"unstructured": "Callejas Rubio JL, Luna Del Castillo JD, de la Hera FJ, Guirao Arrabal E, Colmenero Ruiz M, Ortego CN. Effectiveness of corticoid pulses in patients with cytokine storm syndrome induced by SARS-CoV-2 infection. Med Clin (Barc). 2020;155(4):159–61.",
"volume": "155",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0239401",
"author": "G Ruiz-Irastorza",
"doi-asserted-by": "publisher",
"issue": "9",
"journal-title": "PLoS ONE",
"key": "1201_CR13",
"unstructured": "Ruiz-Irastorza G, Pijoan JI, Bereciartua E, et al. Second week methyl-prednisolone pulses improve prognosis in patients with severe coronavirus disease 2019 pneumonia: an observational comparative study using routine care data. PLoS ONE. 2020;15(9): e0239401.",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"author": "P Horby",
"doi-asserted-by": "publisher",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "1201_CR14",
"unstructured": "Group RC, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17023",
"author": "JAC Sterne",
"doi-asserted-by": "publisher",
"first-page": "1330",
"issue": "13",
"journal-title": "JAMA",
"key": "1201_CR15",
"unstructured": "WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–41.",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1186/s12879-021-06045-3",
"author": "K Ranjbar",
"doi-asserted-by": "publisher",
"first-page": "337",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "1201_CR16",
"unstructured": "Ranjbar K, Moghadami M, Mirahmadizadeh A, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis. 2021;21(1):337.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0252057",
"author": "MA Pinzon",
"doi-asserted-by": "publisher",
"issue": "5",
"journal-title": "PLoS ONE",
"key": "1201_CR17",
"unstructured": "Pinzon MA, Ortiz S, Holguin H, et al. Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS ONE. 2021;16(5): e0252057.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.804250",
"author": "Y Wang",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol",
"key": "1201_CR18",
"unstructured": "Wang Y, Zhu K, Dai R, et al. Specific interleukin-1 inhibitors, specific interleukin-6 inhibitors, and GM-CSF blockades for COVID-19 (at the edge of sepsis): a systematic review. Front Pharmacol. 2021;12: 804250.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2021.800492",
"author": "X Zhang",
"doi-asserted-by": "publisher",
"journal-title": "Front Med (Lausanne).",
"key": "1201_CR19",
"unstructured": "Zhang X, Shang L, Fan G, et al. The efficacy and safety of janus kinase inhibitors for patients with COVID-19: a living systematic review and meta-analysis. Front Med (Lausanne). 2021;8: 800492.",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.2174/1381612824666180123110042",
"author": "C Angelidis",
"doi-asserted-by": "publisher",
"first-page": "659",
"issue": "6",
"journal-title": "Curr Pharm Des",
"key": "1201_CR20",
"unstructured": "Angelidis C, Kotsialou Z, Kossyvakis C, et al. Colchicine pharmacokinetics and mechanism of action. Curr Pharm Des. 2018;24(6):659–63.",
"volume": "24",
"year": "2018"
},
{
"DOI": "10.3389/fimmu.2020.00865",
"author": "A Soriano",
"doi-asserted-by": "publisher",
"first-page": "865",
"journal-title": "Front Immunol",
"key": "1201_CR21",
"unstructured": "Soriano A, Soriano M, Espinosa G, et al. Current therapeutic options for the main monogenic autoinflammatory diseases and PFAPA syndrome: Evidence-based approach and proposal of a practical guide. Front Immunol. 2020;11:865.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1093/rheumatology/keaa217",
"author": "S Piantoni",
"doi-asserted-by": "publisher",
"first-page": "1769",
"issue": "7",
"journal-title": "Rheumatology (Oxford)",
"key": "1201_CR22",
"unstructured": "Piantoni S, Patroni A, Toniati P, et al. Why not to use colchicine in COVID-19? An oldanti-inflammatory drug for a novel auto-inflammatory disease. Rheumatology (Oxford). 2020;59(7):1769–70.",
"volume": "59",
"year": "2020"
},
{
"DOI": "10.1007/s13205-019-1917-z",
"author": "N Lu",
"doi-asserted-by": "publisher",
"first-page": "392",
"issue": "11",
"journal-title": "3 Biotech.",
"key": "1201_CR23",
"unstructured": "Lu N, Yang Y, Liu H, et al. Inhibition of respiratory syncytial virus replication and suppression of RSV-induced airway inflammation in neonatal rats by colchicine. 3 Biotech. 2019;9(11):392.",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1186/s13063-021-05046-6",
"author": "M Salinas",
"doi-asserted-by": "publisher",
"first-page": "92",
"issue": "1",
"journal-title": "Trials",
"key": "1201_CR24",
"unstructured": "Salinas M, Andino P, Palma L, Valencia J, Figueroa E, Ortega J. Early use of corticosteroids in non-critical patients with COVID-19 pneumonia (PREDCOVID): a structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22(1):92.",
"volume": "22",
"year": "2021"
},
{
"key": "1201_CR25",
"unstructured": "WHO R&D Blueprint novel Coronavirus COVID-19 Therapeutic Trial Synopsis: Ordinal Scale for clinical improvement. 2020. Available at: https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis. Accessed 10 Apr 2022."
},
{
"key": "1201_CR26",
"unstructured": "WHO welcomes preliminary results about dexamethasone use in treating critically ill COVID-19 patients. 2020. Available at: https://www.who.int/news/item/16-06-2020-who-welcomes-preliminary-results-about-dexamethasone-use-in-treating-critically-ill-covid-19-patients. Accessed 11 Apr 2022."
},
{
"key": "1201_CR27",
"unstructured": "EMA endorses use of dexamethasone in COVID-19 patients on oxygen or mechanical ventilation—European Medicines Agency. 2020. Available at: https://www.emaeuropa.eu/en/news/ema-endorses-use-dexamethasone-covid-19-patients-oxygen-mechanical-ventilation. Accessed 10 Apr 2022."
},
{
"key": "1201_CR28",
"unstructured": "Steroid Conversion Calculator. 2005. Available at: https://www.mdcalc.com/calc/2040/steroid-conversion-calculator. Accessed 10 Apr 2022."
},
{
"DOI": "10.1042/cs0940557",
"author": "PJ Barnes",
"doi-asserted-by": "publisher",
"first-page": "557",
"issue": "6",
"journal-title": "Clin Sci (Lond)",
"key": "1201_CR29",
"unstructured": "Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond). 1998;94(6):557–72.",
"volume": "94",
"year": "1998"
},
{
"DOI": "10.3389/fimmu.2020.613435",
"author": "S Ronchetti",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "1201_CR30",
"unstructured": "Ronchetti S, Ayroldi E, Ricci E, Gentili M, Migliorati G, Riccardi C. A glance at the use of glucocorticoids in rare inflammatory and autoimmune diseases: Still an indispensable pharmacological tool? Front Immunol. 2020;11: 613435.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1002/j.1552-4604.1996.tb04753.x",
"author": "GM Ferron",
"doi-asserted-by": "publisher",
"first-page": "874",
"issue": "10",
"journal-title": "J Clin Pharmacol",
"key": "1201_CR31",
"unstructured": "Ferron GM, Rochdi M, Jusko WJ, Scherrmann JM. Oral absorption characteristics and pharmacokinetics of colchicine in healthy volunteers after single and multiple doses. J Clin Pharmacol. 1996;36(10):874–83.",
"volume": "36",
"year": "1996"
},
{
"DOI": "10.2165/00003088-197904020-00004",
"author": "ME Pickup",
"doi-asserted-by": "publisher",
"first-page": "111",
"issue": "2",
"journal-title": "Clin Pharmacokinet",
"key": "1201_CR32",
"unstructured": "Pickup ME. Clinical pharmacokinetics of prednisone and prednisolone. Clin Pharmacokinet. 1979;4(2):111–28.",
"volume": "4",
"year": "1979"
},
{
"DOI": "10.1016/j.clim.2020.108490",
"author": "E Della-Torre",
"doi-asserted-by": "publisher",
"journal-title": "Clin Immunol",
"key": "1201_CR33",
"unstructured": "Della-Torre E, Della-Torre F, Kusanovic M, et al. Treating COVID-19 with colchicine in community healthcare setting. Clin Immunol. 2020;217: 108490.",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.3390/jcm9092961",
"author": "L Brunetti",
"doi-asserted-by": "publisher",
"first-page": "2961",
"issue": "9",
"journal-title": "J Clin Med",
"key": "1201_CR34",
"unstructured": "Brunetti L, Diawara O, Tsai A, et al. Colchicine to weather the cytokine storm in hospitalized patients with COVID-19. J Clin Med. 2020;9(9):2961.",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0248276",
"author": "L Manenti",
"doi-asserted-by": "publisher",
"issue": "3",
"journal-title": "PLoS ONE",
"key": "1201_CR35",
"unstructured": "Manenti L, Maggiore U, Fiaccadori E, et al. Reduced mortality in COVID-19 patients treated with colchicine: results from a retrospective, observational study. PLoS ONE. 2021;16(3): e0248276.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.3855/jidc.14924",
"author": "O Karakas",
"doi-asserted-by": "publisher",
"first-page": "57",
"issue": "1",
"journal-title": "J Infect Dev Ctries",
"key": "1201_CR36",
"unstructured": "Karakas O, Erden A, Guven SC, et al. Reducing length of hospital stay with colchicine. J Infect Dev Ctries. 2022;16(1):57–62.",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.1155/2020/8865954",
"author": "T Sandhu",
"doi-asserted-by": "publisher",
"first-page": "8865954",
"journal-title": "Can J Infect Dis Med Microbiol.",
"key": "1201_CR37",
"unstructured": "Sandhu T, Tieng A, Chilimuri S, Franchin G. A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe COVID-19 infection. Can J Infect Dis Med Microbiol. 2020;2020:8865954.",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1136/annrheumdis-2020-217712",
"author": "M Scarsi",
"doi-asserted-by": "publisher",
"first-page": "1286",
"issue": "10",
"journal-title": "Ann Rheum Dis",
"key": "1201_CR38",
"unstructured": "Scarsi M, Piantoni S, Colombo E, et al. Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann Rheum Dis. 2020;79(10):1286–9.",
"volume": "79",
"year": "2020"
},
{
"DOI": "10.1186/s12941-021-00460-9",
"author": "MA Pinzon",
"doi-asserted-by": "publisher",
"first-page": "66",
"issue": "1",
"journal-title": "Ann Clin Microbiol Antimicrob",
"key": "1201_CR39",
"unstructured": "Pinzon MA, Cardona Arango D, Betancur JF, et al. Clinical outcome of patients with COVID-19 Pneumonia treated with corticosteroids and colchicine in Colombia. Ann Clin Microbiol Antimicrob. 2021;20(1):66.",
"volume": "20",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00222-8",
"author": "JC Tardif",
"doi-asserted-by": "publisher",
"first-page": "924",
"issue": "8",
"journal-title": "Lancet Respir Med",
"key": "1201_CR40",
"unstructured": "Tardif JC, Bouabdallaoui N, L’Allier PL, et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021;9(8):924–32.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"author": "SG Deftereos",
"doi-asserted-by": "publisher",
"issue": "6",
"journal-title": "JAMA Netw Open",
"key": "1201_CR41",
"unstructured": "Deftereos SG, Giannopoulos G, Vrachatis DA, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3(6): e2013136.",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1136/rmdopen-2020-001455",
"author": "MI Lopes",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "RMD Open",
"key": "1201_CR42",
"unstructured": "Lopes MI, Bonjorno LP, Giannini MC, et al. Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open. 2021;7(1): e001455.",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.2147/IJGM.S329810",
"author": "DA Pascual-Figal",
"doi-asserted-by": "publisher",
"first-page": "5517",
"journal-title": "Int J Gen Med.",
"key": "1201_CR43",
"unstructured": "Pascual-Figal DA, Roura-Piloto AE, Moral-Escudero E, et al. Colchicine in recently hospitalized patients with COVID-19: a randomized controlled trial (COL-COVID). Int J Gen Med. 2021;14:5517–26.",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1155/2021/2129006",
"author": "M Alsultan",
"doi-asserted-by": "publisher",
"first-page": "2129006",
"journal-title": "Interdiscip Perspect Infect Dis.",
"key": "1201_CR44",
"unstructured": "Alsultan M, Obeid A, Alsamarrai O, et al. Efficacy of colchicine and budesonide in improvement outcomes of patients with coronavirus infection 2019 in Damascus, Syria: a randomized control trial. Interdiscip Perspect Infect Dis. 2021;2021:2129006.",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.18087/cardio.2021.2.n1560",
"author": "VY Mareev",
"doi-asserted-by": "publisher",
"first-page": "15",
"issue": "2",
"journal-title": "Kardiologiia",
"key": "1201_CR45",
"unstructured": "Mareev VY, Orlova YA, Plisyk AG, et al. Proactive anti-inflammatory therapy with colchicine in the treatment of advanced stages of new coronavirus infection. The first results of the COLORIT study. Kardiologiia. 2021;61(2):15–27.",
"volume": "61",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7319",
"author": "G Pourdowlat",
"doi-asserted-by": "publisher",
"first-page": "891",
"issue": "2",
"journal-title": "Phytother Res",
"key": "1201_CR46",
"unstructured": "Pourdowlat G, Saghafi F, Mozafari A, et al. Efficacy and safety of colchicine treatment in patients with COVID-19: a prospective, multicenter, randomized clinical trial. Phytother Res. 2022;36(2):891–8.",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.3399/BJGP.2022.0083",
"author": "J Dorward",
"doi-asserted-by": "publisher",
"first-page": "e446",
"issue": "720",
"journal-title": "Br J Gen Pract",
"key": "1201_CR47",
"unstructured": "Dorward J, Yu LM, Hayward G, et al. Colchicine for COVID-19 in adults in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial. Br J Gen Pract. 2022;72(720):e446–55.",
"volume": "72",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(21)00435-5",
"author": "Recovery Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "1419",
"issue": "12",
"journal-title": "Lancet Respir Med",
"key": "1201_CR48",
"unstructured": "Recovery Collaborative Group. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet Respir Med. 2021;9(12):1419–26.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.41328",
"author": "R Diaz",
"doi-asserted-by": "publisher",
"issue": "12",
"journal-title": "JAMA Netw Open",
"key": "1201_CR49",
"unstructured": "Diaz R, Orlandini A, Castellana N, et al. Effect of colchicine vs usual care alone on intubation and 28-day mortality in patients hospitalized with COVID-19: a randomized clinical trial. JAMA Netw Open. 2021;4(12): e2141328.",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1007/s11606-021-07203-8",
"author": "A Absalon-Aguilar",
"doi-asserted-by": "publisher",
"first-page": "4",
"issue": "1",
"journal-title": "J Gen Intern Med",
"key": "1201_CR50",
"unstructured": "Absalon-Aguilar A, Rull-Gabayet M, Perez-Fragoso A, et al. Colchicine is safe though ineffective in the treatment of severe COVID-19: a randomized clinical trial (COLCHIVID). J Gen Intern Med. 2022;37(1):4–14.",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2021.101242",
"author": "HG Gaitan-Duarte",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine.",
"key": "1201_CR51",
"unstructured": "Gaitan-Duarte HG, Alvarez-Moreno C, Rincon-Rodriguez CJ, et al. Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and combinations thereof in hospitalized patients with COVID-19: a pragmatic, open-label randomized trial. EClinicalMedicine. 2022;43: 101242.",
"volume": "43",
"year": "2022"
},
{
"author": "A Mikolajewska",
"first-page": "CD015045",
"journal-title": "Cochrane Database Syst Rev",
"key": "1201_CR52",
"unstructured": "Mikolajewska A, Fischer AL, Piechotta V, et al. Colchicine for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;10:CD015045.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/j.dsx.2022.102395",
"author": "A Zein",
"doi-asserted-by": "publisher",
"issue": "2",
"journal-title": "Diabetes Metab Syndr",
"key": "1201_CR53",
"unstructured": "Zein A, Raffaello WM. Effect of colchicine on mortality in patients with COVID-19—a systematic review and meta-analysis. Diabetes Metab Syndr. 2022;16(2): 102395.",
"volume": "16",
"year": "2022"
},
{
"author": "CH Lien",
"first-page": "864",
"issue": "8",
"journal-title": "Life (Basel).",
"key": "1201_CR54",
"unstructured": "Lien CH, Lee MD, Weng SL, et al. Repurposing colchicine in treating patients with COVID-19: a systematic review and meta-analysis. Life (Basel). 2021;11(8):864.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1136/rmdopen-2021-001746",
"author": "KG Mehta",
"doi-asserted-by": "publisher",
"issue": "3",
"journal-title": "RMD Open",
"key": "1201_CR55",
"unstructured": "Mehta KG, Patel T, Chavda PD, Patel P. Efficacy and safety of colchicine in COVID-19: a meta-analysis of randomised controlled trials. RMD Open. 2021;7(3): e001746.",
"volume": "7",
"year": "2021"
},
{
"author": "E De-Miguel-Balsa",
"first-page": "7151",
"issue": "22",
"journal-title": "Eur Rev Med Pharmacol Sci",
"key": "1201_CR56",
"unstructured": "De-Miguel-Balsa E, Estevan-Ortega R, Sempere-Selva MT, et al. Can we still consider treatment with colchicine effective in SARS-COV-2 infection? Systematic review, meta-analysis, and trial sequential analysis. Eur Rev Med Pharmacol Sci. 2021;25(22):7151–61.",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1080/07853890.2021.1993327",
"author": "D Sanghavi",
"doi-asserted-by": "publisher",
"first-page": "775",
"issue": "1",
"journal-title": "Ann Med",
"key": "1201_CR57",
"unstructured": "Sanghavi D, Bansal P, Kaur IP, et al. Impact of colchicine on mortality and morbidity in COVID-19: a systematic review. Ann Med. 2022;54(1):775–89.",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1002/iid3.562",
"author": "CS Kow",
"doi-asserted-by": "publisher",
"first-page": "255",
"issue": "2",
"journal-title": "Immun Inflamm Dis.",
"key": "1201_CR58",
"unstructured": "Kow CS, Lee LH, Ramachandram DS, Hasan SS, Ming LC, Goh HP. The effect of colchicine on mortality outcome and duration of hospital stay in patients with COVID-19: A meta-analysis of randomized trials. Immun Inflamm Dis. 2022;10(2):255–64.",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.intimp.2021.107723",
"author": "EN Nawangsih",
"doi-asserted-by": "publisher",
"journal-title": "Int Immunopharmacol",
"key": "1201_CR59",
"unstructured": "Nawangsih EN, Kusmala YY, Rakhmat II, et al. Colchicine and mortality in patients with coronavirus disease 2019 (COVID-19) pneumonia: a systematic review, meta-analysis, and meta-regression. Int Immunopharmacol. 2021;96: 107723.",
"volume": "96",
"year": "2021"
},
{
"DOI": "10.1177/20587384211031763",
"author": "M Golpour",
"doi-asserted-by": "publisher",
"first-page": "205873842110317",
"journal-title": "Int J Immunopathol Pharmacol",
"key": "1201_CR60",
"unstructured": "Golpour M, Mousavi T, Alimohammadi M, et al. The effectiveness of colchicine as an anti-inflammatory drug in the treatment of coronavirus disease 2019: meta-analysis. Int J Immunopathol Pharmacol. 2021;35:20587384211031764.",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1007/s40744-022-00425-0",
"author": "AA Drosos",
"doi-asserted-by": "publisher",
"first-page": "379",
"issue": "2",
"journal-title": "Rheumatol Ther.",
"key": "1201_CR61",
"unstructured": "Drosos AA, Pelechas E, Drossou V, Voulgari PV. Colchicine against SARS-CoV-2 infection: what is the evidence? Rheumatol Ther. 2022;9(2):379–89.",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1111/eci.13645",
"author": "MN Elshafei",
"doi-asserted-by": "publisher",
"issue": "9",
"journal-title": "Eur J Clin Invest",
"key": "1201_CR62",
"unstructured": "Elshafei MN, El-Bardissy A, Khalil A, et al. Colchicine use might be associated with lower mortality in COVID-19 patients: a meta-analysis. Eur J Clin Invest. 2021;51(9): e13645.",
"volume": "51",
"year": "2021"
},
{
"DOI": "10.1111/1440-1681.13488",
"author": "TI Hariyanto",
"doi-asserted-by": "publisher",
"first-page": "823",
"issue": "6",
"journal-title": "Clin Exp Pharmacol Physiol",
"key": "1201_CR63",
"unstructured": "Hariyanto TI, Halim DA, Jodhinata C, Yanto TA, Kurniawan A. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Clin Exp Pharmacol Physiol. 2021;48(6):823–30.",
"volume": "48",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0261358",
"author": "L Chiu",
"doi-asserted-by": "publisher",
"issue": "12",
"journal-title": "PLoS ONE",
"key": "1201_CR64",
"unstructured": "Chiu L, Lo CH, Shen M, et al. Colchicine use in patients with COVID-19: a systematic review and meta-analysis. PLoS ONE. 2021;16(12): e0261358.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1002/jcla.24057",
"author": "M Sarwar",
"doi-asserted-by": "publisher",
"issue": "12",
"journal-title": "J Clin Lab Anal",
"key": "1201_CR65",
"unstructured": "Sarwar M, Ali Z, Fatima M, Sarfraz Z, Sarfraz A, Cherrez-Ojeda I. Colchicine, COVID-19 and hematological parameters: a meta-analysis. J Clin Lab Anal. 2021;35(12): e24057.",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1111/bjh.17885",
"author": "S Abdeen",
"doi-asserted-by": "publisher",
"first-page": "923",
"issue": "4",
"journal-title": "Br J Haematol",
"key": "1201_CR66",
"unstructured": "Abdeen S, Abu-Fanne R, Bdeir K, et al. Divergent impacts of tocilizumab and colchicine in COVID-19-associated coagulopathy: the role of alpha-defensins. Br J Haematol. 2022;196(4):923–7.",
"volume": "196",
"year": "2022"
}
],
"reference-count": 66,
"references-count": 66,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s40261-022-01201-2"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "FRAGILE-COLCOVID19: A Clinical Trial Based on Early Administration of an Oral Combination of Colchicine and Prednisone in Elderly Patients with COVID-19 in Geriatric Facilities",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}
