Efficacy and safety of MAS825 (anti-IL-1β/IL-18) in COVID-19 patients with pneumonia and impaired respiratory function
et al., Clinical and Experimental Immunology, doi:10.1093/cei/uxad065, MAS-COVID, NCT04382651, Jun 2023
RCT 138 hospitalized COVID-19 patients with pneumonia showing no significant difference in mortality or APACHE II scores with MAS825 (anti-IL-1β/IL-18 monoclonal antibody) compared to placebo.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
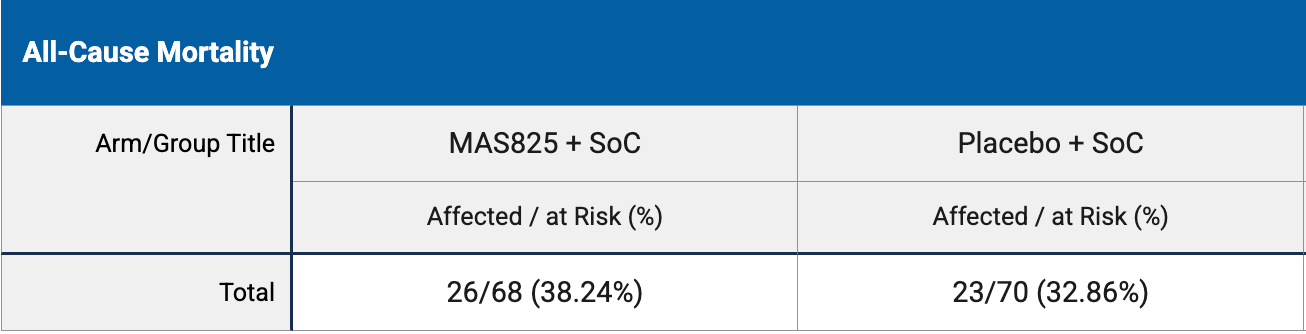
risk of death, 16.4% higher, RR 1.16, p = 0.59, treatment 26 of 68 (38.2%), control 23 of 70 (32.9%).
|
|
APACHE II, 7.4% higher, RR 1.07, p = 0.70, treatment mean 14.5 (±15.4) n=68, control mean 13.5 (±15.1) n=70, day 15.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hakim et al., 20 Jun 2023, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 27 authors, study period 11 June, 2020 - 24 April, 2021, average treatment delay 9.9 days, trial NCT04382651 (history) (MAS-COVID).
Contact: jdmatthews@stcharleshealthcare.org.
Efficacy and safety of MAS825 (anti-IL-1β/IL-18) in COVID-19 patients with pneumonia and impaired respiratory function
Clinical and Experimental Immunology, doi:10.1093/cei/uxad065
MAS825, a bispecific IL-1β/IL-18 monoclonal antibody, could improve clinical outcomes in COVID-19 pneumonia by reducing inflammasomemediated inflammation. Hospitalized non-ventilated patients with COVID-19 pneumonia (n = 138) were randomized (1:1) to receive MAS825 (10 mg/kg single i.v.) or placebo in addition to standard of care (SoC). The primary endpoint was the composite Acute Physiology and Chronic Health Evaluation II (APACHE II) score on Day 15 or on the day of discharge (whichever was earlier) with worst-case imputation for death. Other study endpoints included safety, C-reactive protein (CRP), SARS-CoV-2 presence, and inflammatory markers. On Day 15, the APACHE II score was 14.5 ± 1.87 and 13.5 ± 1.8 in the MAS825 and placebo groups, respectively (P = 0.33). MAS825 + SoC led to 33% relative reduction in intensive care unit (ICU) admissions, ~1 day reduction in ICU stay, reduction in mean duration of oxygen support (13.5 versus 14.3 days), and earlier clearance of virus on Day 15 versus placebo + SoC group. On Day 15, compared with placebo group, patients treated with MAS825 + SoC showed a 51% decrease in CRP levels, 42% lower IL-6 levels, 19% decrease in neutrophil levels, and 16% lower interferon-γ levels, indicative of IL-1β and IL-18 pathway engagement. MAS825 + SoC did not improve APACHE II score in hospitalized patients with severe COVID-19 pneumonia; however, it inhibited relevant clinical and inflammatory pathway biomarkers and resulted in faster virus clearance versus placebo + SoC. MAS825 used in conjunction with SoC was well tolerated. None of the adverse events (AEs) or serious AEs were treatment-related.
Supplementary data Supplementary data is available at Clinical and Experimental Immunology online.
Ethical Approval The study was conducted in accordance ICH GCP and Declaration of Helsinki. Patients or their legal representatives provided signed informed consent. The study was approved by the institutional review board and ethical committees of participating institutes. Additional details have been provided in the manuscript.
Conflict of Interests
Author Contributions The study was designed by G.J., M.K., R.R.S., M.R., R.L., T.S. and F.W.L. A.D.H., M.A., H.O.N., O.S., N.J., R.L., J.O., A.C., T.H., B.P., M.W., G.J.C., A.P., J.W., P.K., T.S., A.P., J.B.P., J.J., G.J., M.K., R.R.S., M.R., R.L., T.S., and F.W.L. contributed to the conduct of these studies. Data were acquired by A.D.H., M.A., H.O.N., O.S., N.J., R.L.,J.O., A.C., T.H., B.P., M.W., G.J.C. and analyzed by A.D.H., G.J., M.K , P.K., M.R., T.S., F.W.L. All authors contributed equally to the interpretation of data. A.D.H. is the principal investigator for this study. All authors contributed to the intellectual content of the manuscript and approved it for publication.
Permission to Reproduce This manuscript does not contain data that requires permission to reproduce.
Clinical Trial Registration ClinicalTrials.gov, NCT04382651
References
Akavipat, Thinkhamrop, Thinkhamrop, Sriraj, Acute physiology and chronic health evaluation (APACHE) II Score-the clinical predictor in neurosurgical intensive care unit, Acta Clin Croat, doi:10.20471/acc.2019.58.01.07
Chiang, Korinek, Cheng, Hwang, Targeting neutrophils to treat acute respiratory distress syndrome in coronavirus disease, Front Pharmacol, doi:10.3389/fphar.2020.572009
Gomez-Rial, Rivero-Calle, Salas, Martinón-Torres, Role of monocytes/macrophages in COVID-19 pathogenesis: Implications for therapy, Infect Drug Resist
Group, Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet
Gustine, Jones, Immunopathology of hyperinflammation in COVID-19, Am J Pathol, doi:10.1016/j.ajpath.2020.08.009
Lescure, Honda, Fowler, Lazar, Shi et al., Sarilumab COVID-19 Global Study Group. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial, Lancet Respir Med, doi:10.1016/S2213-2600(21)00099-0
Lucas, Wong, Klein, Castro, Silva et al., Longitudinal analyses reveal immunological misfiring in severe COVID-19, Nature, doi:10.1038/s41586-020-2588-y
Mann, Menon, Knight, Konkel, Jagger et al., Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19, Sci Immunol, doi:10.1126/sciimmunol.abd6197
Merad, Martin, Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages, Nat Rev Immunol, doi:10.1038/s41577-020-0331-4
Rodrigues, De Sá, Ishimoto, Becerra, Oliveira et al., Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients, J Exp Med, doi:10.1084/jem.20201707
Rosas, Bräu, Waters, Go, Hunter et al., Tocilizumab in hospitalized patients with severe COVID-19 pneumonia, N Engl J Med, doi:10.1056/NEJMoa2028700
Salama, Han, Yau, Reiss, Kramer et al., Tocilizumab in patients hospitalized with COVID-19 pneumonia, N Engl J Med, doi:10.1056/NEJMoa2030340
Schulte-Schrepping, Reusch, Paclik, Baßler, Schlickeiser et al., Deutsche COVID-19 OMICS Initiative (DeCOI). Severe COVID-19 is marked by a dysregulated myeloid cell compartment, Cell, doi:10.1016/j.cell.2020.08.001
Tzotzos, Fischer, Fischer, Zeitlinger, Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey, Crit Care, doi:10.1186/s13054-020-03240-7
Vecchie, Bonaventura, Toldo, Dagna, Dinarello et al., IL-18 and infections: Is there a role for targeted therapies?, J Cell Physiol, doi:10.1002/jcp.30008
Vora, Lieberman, Hao, Inflammasome activation at the crux of severe COVID-19, Nat Rev Immunol
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review, JAMA, doi:10.1001/jama.2020.12839
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
DOI record:
{
"DOI": "10.1093/cei/uxad065",
"ISSN": [
"0009-9104",
"1365-2249"
],
"URL": "http://dx.doi.org/10.1093/cei/uxad065",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>MAS825, a bispecific IL-1β/IL-18 monoclonal antibody, could improve clinical outcomes in COVID-19 pneumonia by reducing inflammasome-mediated inflammation. Hospitalized non-ventilated patients with COVID-19 pneumonia (n = 138) were randomized (1:1) to receive MAS825 (10 mg/kg single i.v.) or placebo in addition to standard of care (SoC). The primary endpoint was the composite Acute Physiology and Chronic Health Evaluation II (APACHE II) score on Day 15 or on the day of discharge (whichever was earlier) with worst-case imputation for death. Other study endpoints included safety, C-reactive protein (CRP), SARS-CoV-2 presence, and inflammatory markers. On Day 15, the APACHE II score was 14.5 ± 1.87 and 13.5 ± 1.8 in the MAS825 and placebo groups, respectively (P = 0.33). MAS825 + SoC led to 33% relative reduction in intensive care unit (ICU) admissions, ~1 day reduction in ICU stay, reduction in mean duration of oxygen support (13.5 versus 14.3 days), and earlier clearance of virus on Day 15 versus placebo + SoC group. On Day 15, compared with placebo group, patients treated with MAS825 + SoC showed a 51% decrease in CRP levels, 42% lower IL-6 levels, 19% decrease in neutrophil levels, and 16% lower interferon-γ levels, indicative of IL-1β and IL-18 pathway engagement. MAS825 + SoC did not improve APACHE II score in hospitalized patients with severe COVID-19 pneumonia; however, it inhibited relevant clinical and inflammatory pathway biomarkers and resulted in faster virus clearance versus placebo + SoC. MAS825 used in conjunction with SoC was well tolerated. None of the adverse events (AEs) or serious AEs were treatment-related.</jats:p>",
"author": [
{
"affiliation": [
{
"name": "Providence Little Company of Mary Medical Center , Torrance, CA , USA"
}
],
"family": "Hakim",
"given": "Alex D",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Dallas Regional Medical Center , Mesquite, TX , USA"
}
],
"family": "Awili",
"given": "Mustafa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Louisiana State University Health Sciences Center and Our Lady of the Lake Regional Medical Center , Baton Rouge, LA , USA"
}
],
"family": "O’Neal",
"given": "Hollis R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Boston Medical Center , Boston, MA , USA"
}
],
"family": "Siddiqi",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rapides Regional Medical Center , Alexandria, LA , USA"
}
],
"family": "Jaffrani",
"given": "Naseem",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of California, Irvine , CA , USA"
}
],
"family": "Lee",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sharp Grossmont Hospital , La Mesa, CA , USA"
}
],
"family": "Overcash",
"given": "Jeffrey S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Louisiana State University Health Sciences Center , Lafayette, LA , USA"
}
],
"family": "Chauffe",
"given": "Ann",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Saint Johns Cancer Institute , Santa Monica, CA , USA"
}
],
"family": "Hammond",
"given": "Terese C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Texas McGovern Medical School , Houston, TX , US"
}
],
"family": "Patel",
"given": "Bela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sharp Chula Vista Medical Center , Chula Vista, CA , USA"
}
],
"family": "Waters",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Thoracic Medicine and Surgery, Lewis Katz School of Medicine at Temple University , Philadelphia, PA , USA"
}
],
"family": "Criner",
"given": "Gerard J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "MorphoSys AG , Boston, MA , USA"
}
],
"family": "Pachori",
"given": "Alok",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Novartis Pharma AG , Basel , Switzerland"
}
],
"family": "Junge",
"given": "Guido",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Novartis Pharma AG , Basel , Switzerland"
}
],
"family": "Levitch",
"given": "Rafael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Oculis , Boston, MA , USA"
}
],
"family": "Watts",
"given": "Jen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Novartis Pharmaceuticals Corporation, East Hanover , NJ , USA"
}
],
"family": "Koo",
"given": "Philip",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Novartis Healthcare Pvt Ltd, Hi-Tech City , Hyderabad , India"
}
],
"family": "Sengupta",
"given": "Tirtha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Novartis Institutes for BioMedical Research , Cambridge, MA , USA"
}
],
"family": "Yu",
"given": "Lili",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Novartis Institutes for BioMedical Research , Basel , Switzerland"
}
],
"family": "Kiffe",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Novartis Institutes for BioMedical Research , Basel , Switzerland"
}
],
"family": "Pinck",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Novartis Institutes for BioMedical Research , Basel , Switzerland"
}
],
"family": "Stein",
"given": "Richard R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Novartis Pharmaceuticals Corporation, East Hanover , NJ , USA"
}
],
"family": "Bendrick-Peart",
"given": "Jamie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Novartis Institutes for BioMedical Research , Cambridge, MA , USA"
}
],
"family": "Jenkins",
"given": "Janet",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Novartis Institutes for BioMedical Research , Cambridge, MA , USA"
}
],
"family": "Rowlands",
"given": "Marianna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Novartis Institutes for BioMedical Research , Cambridge, MA , USA"
}
],
"family": "Waldron-Lynch",
"given": "Frank",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Medicine, St Charles Health System , Bend, OR , USA"
}
],
"family": "Matthews",
"given": "Jesse",
"sequence": "additional"
}
],
"container-title": "Clinical and Experimental Immunology",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
6,
20
]
],
"date-time": "2023-06-20T12:15:29Z",
"timestamp": 1687263329000
},
"deposited": {
"date-parts": [
[
2023,
7,
10
]
],
"date-time": "2023-07-10T11:37:28Z",
"timestamp": 1688989048000
},
"funder": [
{
"name": "Novartis Pharma AG"
}
],
"indexed": {
"date-parts": [
[
2023,
7,
10
]
],
"date-time": "2023-07-10T12:11:05Z",
"timestamp": 1688991065720
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
6,
20
]
]
},
"language": "en",
"link": [
{
"URL": "https://academic.oup.com/cei/advance-article-pdf/doi/10.1093/cei/uxad065/50848525/uxad065.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cei/advance-article-pdf/doi/10.1093/cei/uxad065/50848525/uxad065.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2023,
6,
20
]
]
},
"published-online": {
"date-parts": [
[
2023,
6,
20
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"author": "World Health Organization.",
"key": "2023071011321682100_CIT0001"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "2023071011321682100_CIT0002",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.12839",
"article-title": "Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review",
"author": "Wiersinga",
"doi-asserted-by": "crossref",
"first-page": "782",
"journal-title": "JAMA",
"key": "2023071011321682100_CIT0003",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1186/s13054-020-03240-7",
"article-title": "Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey",
"author": "Tzotzos",
"doi-asserted-by": "crossref",
"first-page": "516",
"journal-title": "Crit Care",
"key": "2023071011321682100_CIT0004",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/j.ajpath.2020.08.009",
"article-title": "Immunopathology of hyperinflammation in COVID-19",
"author": "Gustine",
"doi-asserted-by": "crossref",
"first-page": "4",
"journal-title": "Am J Pathol",
"key": "2023071011321682100_CIT0005",
"volume": "191",
"year": "2021"
},
{
"DOI": "10.1038/s41577-021-00588-x",
"article-title": "Inflammasome activation at the crux of severe COVID-19",
"author": "Vora",
"doi-asserted-by": "crossref",
"first-page": "694",
"journal-title": "Nat Rev Immunol",
"key": "2023071011321682100_CIT0006",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1084/jem.20201707",
"article-title": "Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients",
"author": "Rodrigues",
"doi-asserted-by": "crossref",
"first-page": "e20201707",
"journal-title": "J Exp Med",
"key": "2023071011321682100_CIT0007",
"volume": "218",
"year": "2021"
},
{
"DOI": "10.1002/jcp.30008",
"article-title": "IL-18 and infections: Is there a role for targeted therapies?",
"author": "Vecchie",
"doi-asserted-by": "crossref",
"first-page": "1638",
"journal-title": "J Cell Physiol",
"key": "2023071011321682100_CIT0008",
"volume": "236",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2588-y",
"article-title": "Longitudinal analyses reveal immunological misfiring in severe COVID-19",
"author": "Lucas",
"doi-asserted-by": "crossref",
"first-page": "463",
"journal-title": "Nature",
"key": "2023071011321682100_CIT0009",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.2147/IDR.S258639",
"article-title": "Role of monocytes/macrophages in COVID-19 pathogenesis: Implications for therapy",
"author": "Gomez-Rial",
"doi-asserted-by": "crossref",
"first-page": "2485",
"journal-title": "Infect Drug Resist.",
"key": "2023071011321682100_CIT0010",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.08.001",
"article-title": "Severe COVID-19 is marked by a dysregulated myeloid cell compartment",
"author": "Schulte-Schrepping",
"doi-asserted-by": "crossref",
"first-page": "1419",
"journal-title": "Cell",
"key": "2023071011321682100_CIT0011",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1038/s41577-020-0331-4",
"article-title": "Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages",
"author": "Merad",
"doi-asserted-by": "crossref",
"first-page": "355",
"journal-title": "Nat Rev Immunol",
"key": "2023071011321682100_CIT0012",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2020.572009",
"article-title": "Targeting neutrophils to treat acute respiratory distress syndrome in coronavirus disease",
"author": "Chiang",
"doi-asserted-by": "crossref",
"first-page": "572009",
"journal-title": "Front Pharmacol",
"key": "2023071011321682100_CIT0013",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1126/sciimmunol.abd6197",
"article-title": "Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19",
"author": "Mann",
"doi-asserted-by": "crossref",
"first-page": "eabd6197",
"journal-title": "Sci Immunol",
"key": "2023071011321682100_CIT0014",
"volume": "5",
"year": "2020"
},
{
"article-title": "Acute physiology and chronic health evaluation (APACHE) II Score—the clinical predictor in neurosurgical intensive care unit",
"author": "Akavipat",
"first-page": "50",
"journal-title": "Acta Clin Croat.",
"key": "2023071011321682100_CIT0015",
"volume": "58",
"year": "2019"
},
{
"DOI": "10.1056/NEJMoa2030340",
"article-title": "Tocilizumab in patients hospitalized with COVID-19 pneumonia",
"author": "Salama",
"doi-asserted-by": "crossref",
"first-page": "20",
"journal-title": "N Engl J Med",
"key": "2023071011321682100_CIT0016",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2028700",
"article-title": "Tocilizumab in hospitalized patients with severe COVID-19 pneumonia",
"author": "Rosas",
"doi-asserted-by": "crossref",
"first-page": "1503",
"journal-title": "N Engl J Med",
"key": "2023071011321682100_CIT0017",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00099-0",
"article-title": "Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial",
"author": "Lescure",
"doi-asserted-by": "crossref",
"first-page": "522",
"journal-title": "Lancet Respir Med.",
"key": "2023071011321682100_CIT0018",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Group RC",
"doi-asserted-by": "crossref",
"first-page": "1637",
"journal-title": "Lancet",
"key": "2023071011321682100_CIT0019",
"volume": "397",
"year": "2021"
}
],
"reference-count": 19,
"references-count": 19,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cei/advance-article/doi/10.1093/cei/uxad065/7203697"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Immunology",
"Immunology and Allergy"
],
"subtitle": [],
"title": "Efficacy and safety of MAS825 (anti-IL-1β/IL-18) in COVID-19 patients with pneumonia and impaired respiratory function",
"type": "journal-article"
}