Factors Influencing Disease Stability and Response to Tocilizumab Therapy in Severe COVID-19: A Retrospective Cohort Study
et al., Antibiotics, doi:10.3390/antibiotics11081078, Aug 2022
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
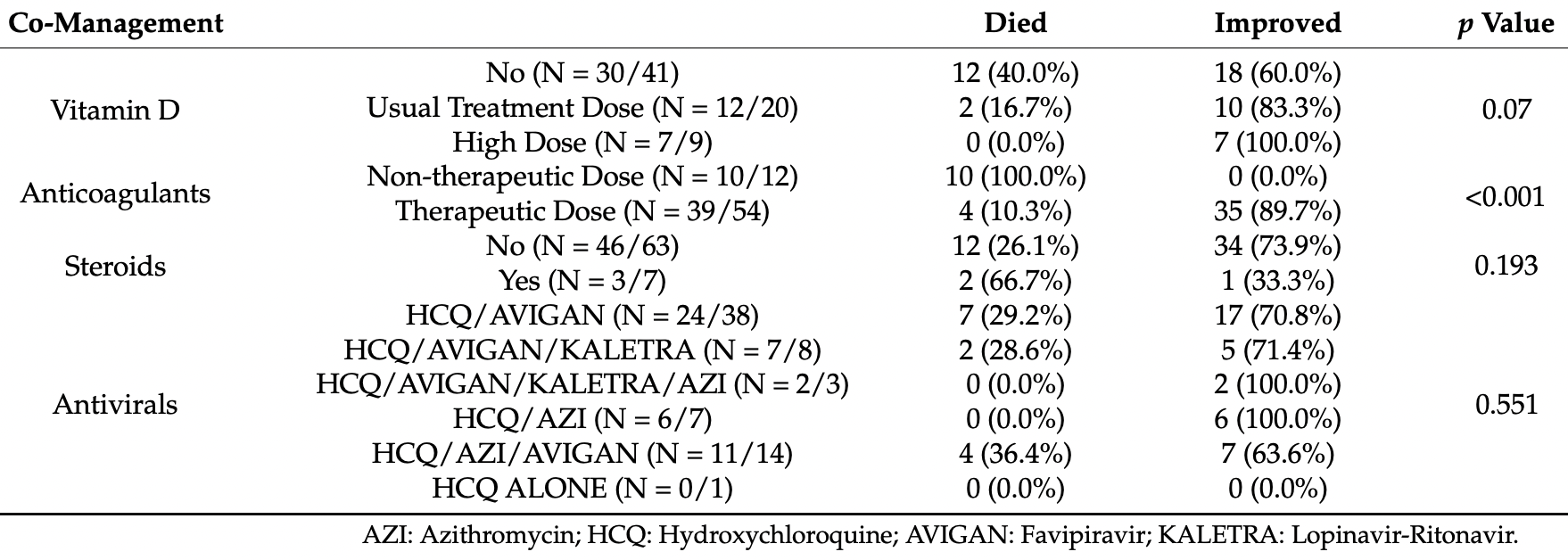
Retrospective 49 severe COVID-19 patients treated with tocilizumab, showing lower mortality with vitamin D treatment and a dose-dependent response.
Cholecalciferol was used in this study.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 44% [33‑53%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
This is the 98th of 136 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
40 studies are RCTs, which show efficacy with p=0.0000001.
|
risk of death, 93.7% lower, RR 0.06, p = 0.07, treatment 0 of 7 (0.0%), control 12 of 30 (40.0%), NNT 2.5, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), high dose, 50,000IU every other day for two weeks or one intramuscular shot of 300,000IU.
|
|
risk of death, 58.3% lower, RR 0.42, p = 0.28, treatment 2 of 12 (16.7%), control 12 of 30 (40.0%), NNT 4.3, low dose, ≤10,000IU/day.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hafez et al., 9 Aug 2022, retrospective, Egypt, peer-reviewed, 2 authors, study period April 2020 - June 2020, dosage 50,000IU days 1, 3, 5, 7, 9, 11, 13, 50,000IU every other day for two weeks or one intramuscular shot of 300,000IU.
Contact: wael.hafez@nmc.ae (corresponding author).
Factors Influencing Disease Stability and Response to Tocilizumab Therapy in Severe COVID-19: A Retrospective Cohort Study
Antibiotics, doi:10.3390/antibiotics11081078
1) Background: The efficacy of tocilizumab in COVID-19 has been doubted. The study aimed to investigate factors affecting disease stability and response to tocilizumab among severe COVID-19 patients. (2) Methods: This was a cohort study of 70 severe COVID-19 patients at NMC Royal Hospital, UAE, from April to June 2020. (3) Results: Elderly patients and those with cardiovascular comorbidities had a higher risk of unstable COVID-19 (p = 0.025). Regarding tocilizumab therapy timing, compared to the critical group receiving tocilizumab, the unstable severe patients receiving tocilizumab had a significantly higher rate of improvement (86%). In contrast, the late critical subgroup showed a significantly increased mortality rate (52.9%). The risk for secondary infection and adverse events following tocilizumab was higher in the late critical group than in the unstable severe and early critical groups (p = 0.024 and p = 0.006, respectively). Therapeutic doses of anticoagulation and high-dose vitamin D were correlated with better outcomes than the prophylactic dose and the treatment dose of vitamin D (p < 0.001 and p = 0.07, respectively). (4) Conclusions: elderly patients and those with cardiovascular disease developed unstable COVID-19. Tocilizumab is a potentially effective choice against severe and critical COVID-19. Early tocilizumab administration combined with therapeutic dose anticoagulation and high vitamin D doses could improve the patients' outcomes.
Informed Consent Statement: This was a retrospective study; all Patients Identifiers were removed during the data collection process, with complete protection of patients' privacy. The study followed the Helsinki Declaration in terms of patient privacy. The Regional Research Ethics Committee, Department of Health, Abu Dhabi, UAE, reviewed and approved the study (Ref: ADHRTC-2021-178). As a retrospective study, informed consent was not required.
Conflicts of Interest: All authors declare no conflict of interest.
References
Abaleke, Abbas, Abbasi, Abbott, Abdelaziz et al., Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial, Lancet, doi:10.1016/S0140-6736(21)00149-5
Abani, Abbas, Abbas, Abbas, Abbasi et al., Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial, Lancet, doi:10.1016/S0140-6736(21)00676-0
Apea, Wan, Dhairyawan, Puthucheary, Pearse et al., Ethnicity and outcomes in patients hospitalised with COVID-19 infection in East London: An observational cohort study, BMJ Open, doi:10.1136/bmjopen-2020-042140
Bartoletti, Azap, Barac, Bussini, Ergonul et al., ESCMID COVID-19 living guidelines: Drug treatment and clinical management, Clin. Microbiol. Infect, doi:10.1016/j.cmi.2021.11.007
Channappanavar, Perlman, Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology, Semin. Immunopathol, doi:10.1007/s00281-017-0629-x
Furlow, COVACTA trial raises questions about tocilizumab's benefit in COVID-19, Lancet. Rheumatol, doi:10.1016/S2665-9913(20)30313-1
Guan, Ni, Hu, Liang, Ou et al., Clinical Characteristics of Coronavirus Disease 2019 in China, N. Engl. J. Med. Orig, doi:10.1056/NEJMoa2002032
Guaraldi, Meschiari, Cozzi-Lepri, Milic, Tonelli et al., Tocilizumab in patients with severe COVID-19: A retrospective cohort study, Lancet Rheumatol
Inciardi, Adamo, Lupi, Cani, Di Pasquale et al., Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy, Eur. Heart J, doi:10.1093/eurheartj/ehaa388
Lan, Lai, Huang, Chang, Lu et al., Tocilizumab for severe COVID-19: A systematic review and meta-analysis, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.106103
Liu, Li, Zhou, Guan, Xiang, Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)?, J. Autoimmun, doi:10.1016/j.jaut.2020.102452
Lu, Chen, Lee, Chang, Potential therapeutic agents against COVID-19: What we know so far, J. Chin. Med. Assoc, doi:10.1097/JCMA.0000000000000318
Luo, Liu, Qiu, Liu, Liu et al., Tocilizumab treatment in COVID-19: A single center experience, J. Med. Virol, doi:10.1002/jmv.25801
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: Consider cytokine storm syndromes and immunosuppression, Lancet, doi:10.1016/S0140-6736(20)30628-0
Michot, Albiges, Chaput, Saada, Pommeret et al., Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: A case report, Ann. Oncol. Off. J. Eur. Soc. Med. Oncol, doi:10.1016/j.annonc.2020.03.300
Munshi, Hussein, Toraih, Elshazli, Jardak et al., Vitamin D insufficiency as a potential culprit in critical COVID-19 patients, J. Med. Virol, doi:10.1002/jmv.26360
Norelli, Camisa, Barbiera, Falcone, Purevdorj et al., Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells, Nat. Med, doi:10.1038/s41591-018-0036-4
Nugroho, Suryantoro, Yuliasih, Rosyid, Asmarawati et al., Optimal use of tocilizumab for severe and critical COVID-19: A systematic review and meta-analysis, doi:10.12688/f1000research.45046.1
Oliynyk, Barg, Oliynyk, Dubrov, Gurianov et al., Lack of Difference in Tocilizumab Efficacy in the Treatment of Severe COVID-19 Caused by Different SARS-CoV-2 Variants, J. Pers. Med, doi:10.3390/jpm12071103
Ortiz-Martínez, Tocilizumab: A new opportunity in the possible therapeutic arsenal against COVID-19, Travel Med. Infect. Dis, doi:10.1016/j.tmaid.2020.101678
Radujkovic, Hippchen, Tiwari-Heckler, Dreher, Boxberger et al., Vitamin D Deficiency and Outcome of COVID-19 Patients, Nutrients, doi:10.3390/nu12092757
Ramaswamy, Mannam, Comer, Pharmd, Brent et al., Off-Label Real World Experience Using Tocilizumab for Patients Hospitalized with COVID-19 Disease in a Regional Community Health System: A Case-Control Study, doi:10.1101/2020.05.14.20099234
Rossotti, Travi, Ughi, Corradin, Baiguera et al., Safety and efficacy of anti-il6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: A comparative analysis, J. Infect, doi:10.1016/j.jinf.2020.07.008
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID -19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Med, doi:10.1007/s00134-020-05991-x
Rubini, Interleukin-6 and lung inflammation: Evidence for a causative role in inducing respiratory system resistance increments, Inflamm. Allergy Drug Targets, doi:10.2174/1871528111312050003
Shankar-Hari, Vale, Godolphin, Fisher, Higgins et al., Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis, JAMA, doi:10.1001/JAMA.2021.11330
Singhal, A Review of Coronavirus Disease-2019 (COVID-19), Indian J. Pediatr, doi:10.1007/s12098-020-03263-6
Toniati, Piva, Cattalini, Garrafa, Regola et al., Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy, Autoimmun. Rev, doi:10.1016/j.autrev.2020.102568
Uciechowski, Dempke, Interleukin-6: A Masterplayer in the Cytokine Network, Oncology, doi:10.1159/000505099
Xu, Shi, Wang, Zhang, Huang et al., Pathological findings of COVID-19 associated with acute respiratory distress syndrome, Lancet Respir. Med, doi:10.1016/S2213-2600(20)30076-X
Zhang, Wu, Wang, Yue, Song et al., Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-kappaB, Virology, doi:10.1016/j.virol.2007.04.009
Zhang, Xiao, Zhang, Xia, Cao et al., Coagulopathy and Antiphospholipid Antibodies in Patients with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMc2007575
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.3390/antibiotics11081078",
"ISSN": [
"2079-6382"
],
"URL": "http://dx.doi.org/10.3390/antibiotics11081078",
"abstract": "<jats:p>(1) Background: The efficacy of tocilizumab in COVID-19 has been doubted. The study aimed to investigate factors affecting disease stability and response to tocilizumab among severe COVID-19 patients. (2) Methods: This was a cohort study of 70 severe COVID-19 patients at NMC Royal Hospital, UAE, from April to June 2020. (3) Results: Elderly patients and those with cardiovascular comorbidities had a higher risk of unstable COVID-19 (p = 0.025). Regarding tocilizumab therapy timing, compared to the critical group receiving tocilizumab, the unstable severe patients receiving tocilizumab had a significantly higher rate of improvement (86%). In contrast, the late critical subgroup showed a significantly increased mortality rate (52.9%). The risk for secondary infection and adverse events following tocilizumab was higher in the late critical group than in the unstable severe and early critical groups (p = 0.024 and p = 0.006, respectively). Therapeutic doses of anticoagulation and high-dose vitamin D were correlated with better outcomes than the prophylactic dose and the treatment dose of vitamin D (p < 0.001 and p = 0.07, respectively). (4) Conclusions: elderly patients and those with cardiovascular disease developed unstable COVID-19. Tocilizumab is a potentially effective choice against severe and critical COVID-19. Early tocilizumab administration combined with therapeutic dose anticoagulation and high vitamin D doses could improve the patients’ outcomes.</jats:p>",
"alternative-id": [
"antibiotics11081078"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1203-0808",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hafez",
"given": "Wael",
"sequence": "first"
},
{
"affiliation": [],
"family": "Abdelrahman",
"given": "Ahmed",
"sequence": "additional"
}
],
"container-title": "Antibiotics",
"container-title-short": "Antibiotics",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
10
]
],
"date-time": "2022-08-10T06:42:53Z",
"timestamp": 1660113773000
},
"deposited": {
"date-parts": [
[
2022,
8,
10
]
],
"date-time": "2022-08-10T06:46:58Z",
"timestamp": 1660114018000
},
"indexed": {
"date-parts": [
[
2022,
8,
10
]
],
"date-time": "2022-08-10T07:13:28Z",
"timestamp": 1660115608440
},
"is-referenced-by-count": 0,
"issue": "8",
"issued": {
"date-parts": [
[
2022,
8,
9
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2022,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
9
]
],
"date-time": "2022-08-09T00:00:00Z",
"timestamp": 1660003200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2079-6382/11/8/1078/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1078",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
8,
9
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
9
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1007/s12098-020-03263-6",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1016/S2213-2600(20)30076-X",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1097/JCMA.0000000000000318",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1159/000505099",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1038/s41591-018-0036-4",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106103",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1016/j.jaut.2020.102452",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1002/jmv.25801",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1016/j.autrev.2020.102568",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1101/2020.05.14.20099234",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1016/j.tmaid.2020.101678",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1016/S2665-9913(20)30313-1",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/S0140-6736(21)00149-5",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1007/s00281-017-0629-x",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1016/j.virol.2007.04.009",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1016/j.annonc.2020.03.300",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/S2665-9913(20)30173-9",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1056/NEJMoa2100433",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1016/j.cmi.2021.11.007",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1016/j.jinf.2020.07.008",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1056/NEJMc2007575",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.12688/f1000research.45046.1",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1007/s00134-020-05991-x",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.2174/1871528111312050003",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1001/JAMA.2021.11330",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.3390/nu12092757",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1002/jmv.26360",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1093/eurheartj/ehaa388",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1136/bmjopen-2020-042140",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.3390/jpm12071103",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"key": "ref35",
"unstructured": "Clinical Managment of COVID-19—Intterim Guidance\nhttps://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiD8On8jID3AhVDXRoKHcrIBOgQFnoECAsQAQ&url=https%3A%2F%2Fapps.who.int%2Firis%2Fbitstream%2Fhandle%2F10665%2F332196%2FWHO-2019-nCoV-clinical-2020.5-eng.pdf&usg=AOvVaw3KM"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2079-6382/11/8/1078"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Infectious Diseases",
"Microbiology (medical)",
"General Pharmacology, Toxicology and Pharmaceutics",
"Biochemistry",
"Microbiology"
],
"subtitle": [],
"title": "Factors Influencing Disease Stability and Response to Tocilizumab Therapy in Severe COVID-19: A Retrospective Cohort Study",
"type": "journal-article",
"volume": "11"
}
