Siltuximab downregulates interleukin-8 and pentraxin 3 to improve ventilatory status and survival in severe COVID-19
et al., Leukemia, doi:10.1038/s41375-021-01299-x, May 2021
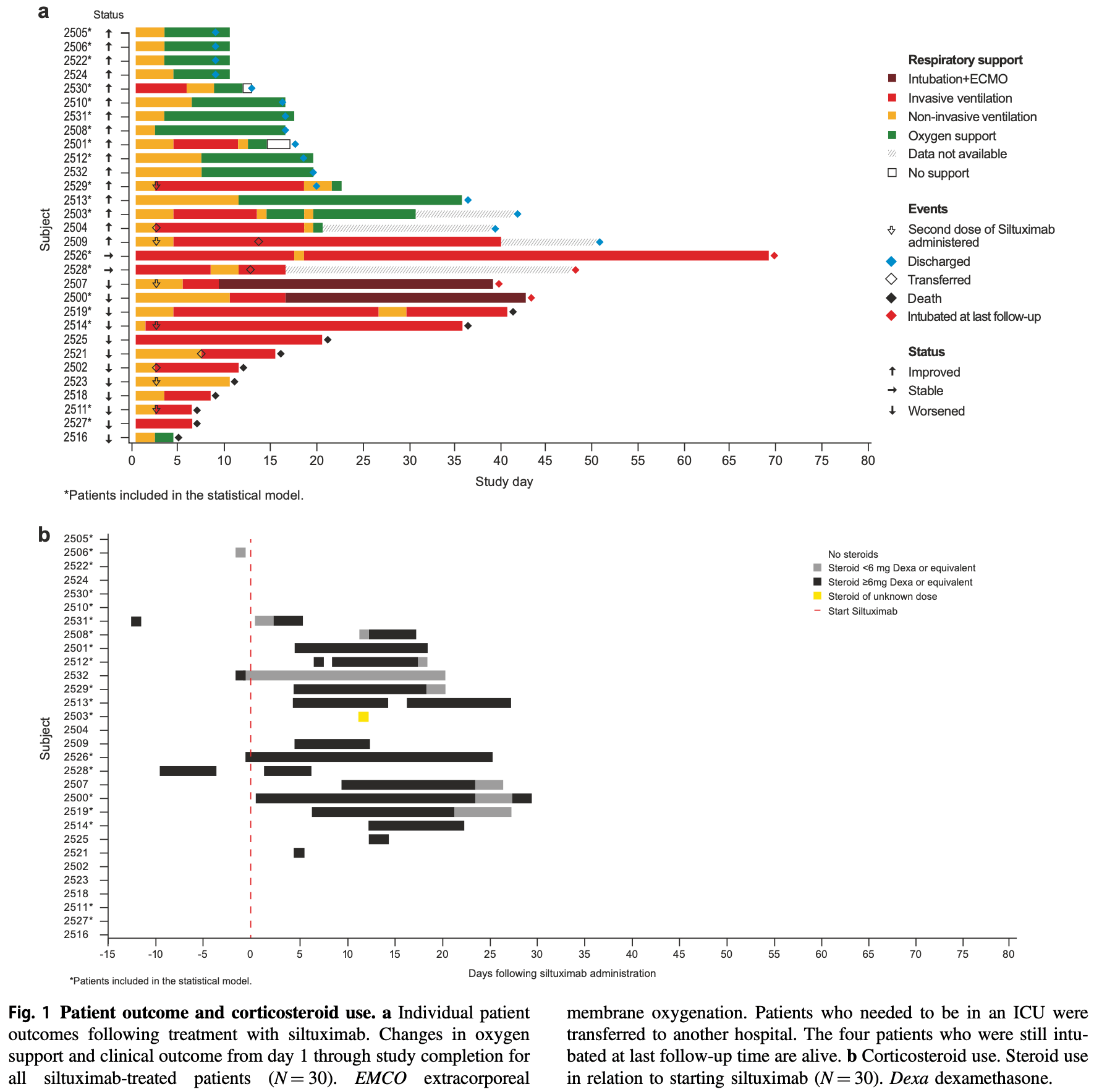
Prospective observational study of 30 hospitalized COVID-19 patients requiring ventilatory support in Italy, showing improved ventilatory status and survival with siltuximab treatment, particularly with the reduction of IL-8 and PTX3 levels 4 days after treatment. There was no control group.
Gritti et al., 24 May 2021, retrospective, Italy, peer-reviewed, 22 authors.
Contact: g.gritti@asst-pg23.it.
Abstract: Leukemia (2021) 35:2710–2714
https://doi.org/10.1038/s41375-021-01299-x
LETTER
INFECTIOUS MEDICINE, VIROLOGY
Siltuximab downregulates interleukin-8 and pentraxin 3 to improve
ventilatory status and survival in severe COVID-19
Giuseppe Gritti 1 Federico Raimondi2,3 Barbara Bottazzi4 Diego Ripamonti5 Ivano Riva6 Francesco Landi1,7
Leonardo Alborghetti5 Marco Frigeni1 Marianna Damiani6,8 Caterina Micò1 Stefano Fagiuoli9
Ferdinando Luca Lorini6 Lucia Gandini6,8 Luca Novelli 2 Jonathan P. Morgan10 Benjamin M. J. Owens10
Karan J. K. Kanhai10 Gordana Tonkovic Reljanovic11 Marco Rizzi5 Fabiano Di Marco 2,12
Alberto Mantovani3,13,14 Alessandro Rambaldi 1,15
●
●
●
●
●
●
●
●
●
●
●
●
●
●
●
●
●
●
●
●
●
Received: 1 April 2021 / Accepted: 12 May 2021 / Published online: 24 May 2021
© The Author(s) 2021. This article is published with open access
1234567890();,:
1234567890();,:
To the Editor:
Severe coronavirus disease 2019 (COVID-19) is characterized by interstitial pneumonia/acute respiratory distress
syndrome and hyperinflammation, with elevated levels of
proinflammatory cytokines, such as interleukin-6 (IL-6),
associated with mortality and patients requiring ventilator
support [1–3]. Targeting the IL-6 signaling pathway has
been identified as a potential strategy to mitigate the elevated cytokines and resulting hyperinflammation associated
with COVID-19 [1]. Siltuximab is the first and only US
Food and Drug Administration- and European Medicines
Agency-approved monoclonal antibody that specifically
binds to IL-6, thereby inactivating IL-6–induced signaling.
Supplementary information The online version contains
supplementary material available at https://doi.org/10.1038/s41375021-01299-x.
Siltuximab is currently approved for the treatment of adults
with idiopathic multicentric Castleman disease [4]. The aim
of this study was to examine the association between siltuximab treatment, serum cytokine and chemokine levels,
and mortality and/or respiratory function in hospitalized
patients with COVID-19 and acute respiratory distress
syndrome.
We designed a prospective, observational cohort study at
the start of the pandemic in response to the urgent unmet need
for an effective treatment for patients with SARS-CoV-2
pneumonia, hyperinflammation, and respiratory failure [5]. In
accordance with clinical guidelines developed at the
Papa Giovanni XXIII Hospital in Bergamo, Italy, siltuximab
was initially supplied under a compassionate-use program
for the emergency treatment of 30 patients with severe
COVID-19 requiring ventilatory support. Consequently, an
investigator-initiated study protocol was developed for
immediate implementation. The study protocol was submitted
and approved through the Hospital Ethics Board. Patients, or
* Giuseppe Gritti
g.gritti@asst-pg23.it
8
Postgraduate School of Anesthesiology and Intensive Care,
University of Milan, Milan, Italy
1
Hematology Unit, Azienda Socio Sanitario Territoriale Papa
Giovanni XXIII, Bergamo, Italy
9
Gastroenterology Unit, Azienda Socio Sanitario Territoriale Papa
Giovanni XXIII, Bergamo, Italy
2
Pneumology Unit, Azienda Socio Sanitario Territoriale Papa
Giovanni XXIII, Bergamo, Italy
10
EUSA Pharma, Hemel Hempstead, UK
11
ErgoMed PLC, Guildford, UK
Postgraduate School of Respiratory Medicine, University of
Milan, Milan, Italy
12
Department of Health Sciences, University of Milan, Milan, Italy
13
Department of Biomedical Sciences, Humanitas University,
Milan,..
DOI record:
{
"DOI": "10.1038/s41375-021-01299-x",
"ISSN": [
"0887-6924",
"1476-5551"
],
"URL": "http://dx.doi.org/10.1038/s41375-021-01299-x",
"alternative-id": [
"1299"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "1 April 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "12 May 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "24 May 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Date",
"name": "change_date",
"order": 4,
"value": "28 June 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Type",
"name": "change_type",
"order": 5,
"value": "Correction"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Details",
"name": "change_details",
"order": 6,
"value": "A Correction to this paper has been published:"
},
{
"URL": "https://doi.org/10.1038/s41375-021-01329-8",
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Details",
"name": "change_details",
"order": 7,
"value": "https://doi.org/10.1038/s41375-021-01329-8"
},
{
"group": {
"label": "Compliance with ethical standards",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "JPM, BMJO, and KJKK are employees of EUSA Pharma. AR reports consultancy fees from Amgen, Celgene, Gilead, Italfarmaco, Novartis, Omeros, Pfizer, and Roche; travel support from Amgen, Celgene, Gilead, Italfarmaco, Novartis, and Roche; and research grants from Amgen, Italfarmaco, and Roche. CM reports travel support from Medac. DR reports personal fees from Gilead, Janssen, and ViiV. FDM reports grants from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis; and personal fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Guidotti/Malesci, Menarini, Mundipharma, Novartis, Teva, and Zambon. GG reports non-financial support from Gilead Kite, Janssen, Roche, and Takeda; and personal fees from Amgen, Autolus, Gilead Kite, Italfarmaco, IQVIA, Roche, and Takeda. IR reports travel support from Aferetica. SF reports grants from Gilead and Novartis; and personal fees from AbbVie, Astellas, Bayer, Gilead, Intercept, Kedrion, Merck Sharp & Dohme, and Novartis. BB and AM are inventors of patents on PTX3 and obtain royalties on related reagents. The other authors declare no competing interests."
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "We complied with all relevant ethical rules. The protocol and informed consent forms were approved by the Hospital Ethics Board at the Papa Giovanni XXIII Hospital in Bergamo, Italy. Patients, or their legal representative, provided either verbal or written consent to participate in the study."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6789-5123",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gritti",
"given": "Giuseppe",
"sequence": "first"
},
{
"affiliation": [],
"family": "Raimondi",
"given": "Federico",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bottazzi",
"given": "Barbara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ripamonti",
"given": "Diego",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Riva",
"given": "Ivano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Landi",
"given": "Francesco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alborghetti",
"given": "Leonardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frigeni",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Damiani",
"given": "Marianna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Micò",
"given": "Caterina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fagiuoli",
"given": "Stefano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lorini",
"given": "Ferdinando Luca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gandini",
"given": "Lucia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2705-248X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Novelli",
"given": "Luca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morgan",
"given": "Jonathan P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Owens",
"given": "Benjamin M. J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kanhai",
"given": "Karan J. K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reljanovic",
"given": "Gordana Tonkovic",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rizzi",
"given": "Marco",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1743-0504",
"affiliation": [],
"authenticated-orcid": false,
"family": "Di Marco",
"given": "Fabiano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mantovani",
"given": "Alberto",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3739-7502",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rambaldi",
"given": "Alessandro",
"sequence": "additional"
}
],
"container-title": "Leukemia",
"container-title-short": "Leukemia",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
5,
24
]
],
"date-time": "2021-05-24T10:02:53Z",
"timestamp": 1621850573000
},
"deposited": {
"date-parts": [
[
2021,
9,
1
]
],
"date-time": "2021-09-01T09:10:56Z",
"timestamp": 1630487456000
},
"indexed": {
"date-parts": [
[
2024,
8,
5
]
],
"date-time": "2024-08-05T12:40:10Z",
"timestamp": 1722861610794
},
"is-referenced-by-count": 35,
"issue": "9",
"issued": {
"date-parts": [
[
2021,
5,
24
]
]
},
"journal-issue": {
"issue": "9",
"published-print": {
"date-parts": [
[
2021,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
24
]
],
"date-time": "2021-05-24T00:00:00Z",
"timestamp": 1621814400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
24
]
],
"date-time": "2021-05-24T00:00:00Z",
"timestamp": 1621814400000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41375-021-01299-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41375-021-01299-x",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41375-021-01299-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "2710-2714",
"prefix": "10.1038",
"published": {
"date-parts": [
[
2021,
5,
24
]
]
},
"published-online": {
"date-parts": [
[
2021,
5,
24
]
]
},
"published-print": {
"date-parts": [
[
2021,
9
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.healun.2020.03.012",
"author": "HK Siddiqi",
"doi-asserted-by": "publisher",
"first-page": "405",
"journal-title": "J Heart Lung Transpl",
"key": "1299_CR1",
"unstructured": "Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transpl. 2020;39:405–7.",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"author": "P Mehta",
"doi-asserted-by": "publisher",
"first-page": "1033",
"journal-title": "Lancet.",
"key": "1299_CR2",
"unstructured": "Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1002/JLB.3COVR0520-272R",
"author": "J Wang",
"doi-asserted-by": "publisher",
"first-page": "17",
"journal-title": "J Leukoc Biol",
"key": "1299_CR3",
"unstructured": "Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108:17–41.",
"volume": "108",
"year": "2020"
},
{
"key": "1299_CR4",
"unstructured": "SYLVANT 100 mg powder for concentrate for solution for infusion – Summary of Product Characteristics (SmPC) – (emc). https://www.medicines.org.uk/emc/product/2132/smpc."
},
{
"DOI": "10.1001/jama.2020.5394",
"author": "G Grasselli",
"doi-asserted-by": "publisher",
"first-page": "1574",
"journal-title": "JAMA",
"key": "1299_CR5",
"unstructured": "Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020;323:1574–81.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1101/2020.04.01.20048561",
"doi-asserted-by": "publisher",
"key": "1299_CR6",
"unstructured": "Gritti G, Raimondi F, Ripamonti D, Riva I, Landi F, Alborghetti L, et al. IL-6 signalling pathway inactivation with siltuximab in patients with COVID-19 respiratory failure: an observational cohort study. medRxiv. 2020. https://doi.org/10.1101/2020.04.01.20048561."
},
{
"DOI": "10.1038/s41590-020-00832-x",
"author": "E Brunetta",
"doi-asserted-by": "publisher",
"first-page": "19",
"journal-title": "Nat Immunol",
"key": "1299_CR7",
"unstructured": "Brunetta E, Folci M, Bottazzi B, De Santis M, Gritti G, Protti A, et al. Macrophage expression and prognostic significance of the long pentraxin PTX3 in COVID-19. Nat Immunol. 2021;22:19–24.",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2020.10.027",
"author": "A Schirinzi",
"doi-asserted-by": "publisher",
"first-page": "84",
"journal-title": "J Infect",
"key": "1299_CR8",
"unstructured": "Schirinzi A, Pesce F, Laterza R, D’Alise MG, Lovero R, Fontana A, et al. Pentraxin 3: Potential prognostic role in SARS-CoV-2 patients admitted to the emergency department. J Infect. 2021;82:84–123.",
"volume": "82",
"year": "2021"
},
{
"DOI": "10.1038/s41591-020-1051-9",
"author": "DM Del Valle",
"doi-asserted-by": "publisher",
"first-page": "1636",
"journal-title": "Nat Med.",
"key": "1299_CR9",
"unstructured": "Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature helps predict COVID-19 severity and death. Nat Med. 2020;26:1636–43.",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1182/bloodadvances.2020003568",
"author": "ML Meizlish",
"doi-asserted-by": "publisher",
"first-page": "1164",
"journal-title": "Blood Adv",
"key": "1299_CR10",
"unstructured": "Meizlish ML, Pine AB, Bishai JD, Goshua G, Nadelmann ER, Simonov M, et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv. 2021;5:1164–77.",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1016/S1470-2045(14)70319-5",
"author": "F van Rhee",
"doi-asserted-by": "publisher",
"first-page": "966",
"journal-title": "Lancet Oncol",
"key": "1299_CR11",
"unstructured": "van Rhee F, Wong RS, Munshi N, Rossi JF, Ke XY, Fosså A, et al. Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2014;15:966–74.",
"volume": "15",
"year": "2014"
}
],
"reference-count": 11,
"references-count": 11,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2020.04.01.20048561",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41375-021-01299-x"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Siltuximab downregulates interleukin-8 and pentraxin 3 to improve ventilatory status and survival in severe COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"updated-by": [
{
"DOI": "10.1038/s41375-021-01329-8",
"label": "Correction",
"type": "correction",
"updated": {
"date-parts": [
[
2021,
6,
28
]
],
"date-time": "2021-06-28T00:00:00Z",
"timestamp": 1624838400000
}
}
],
"volume": "35"
}