ABX464 (obefazimod) for patients with COVID-19 at risk for severe disease: miR-AGE, a randomized, double-blind placebo-controlled trial
et al., Journal of Allergy and Clinical Immunology: Global, doi:10.1016/j.jacig.2023.100140, miR-AGE, Nov 2023
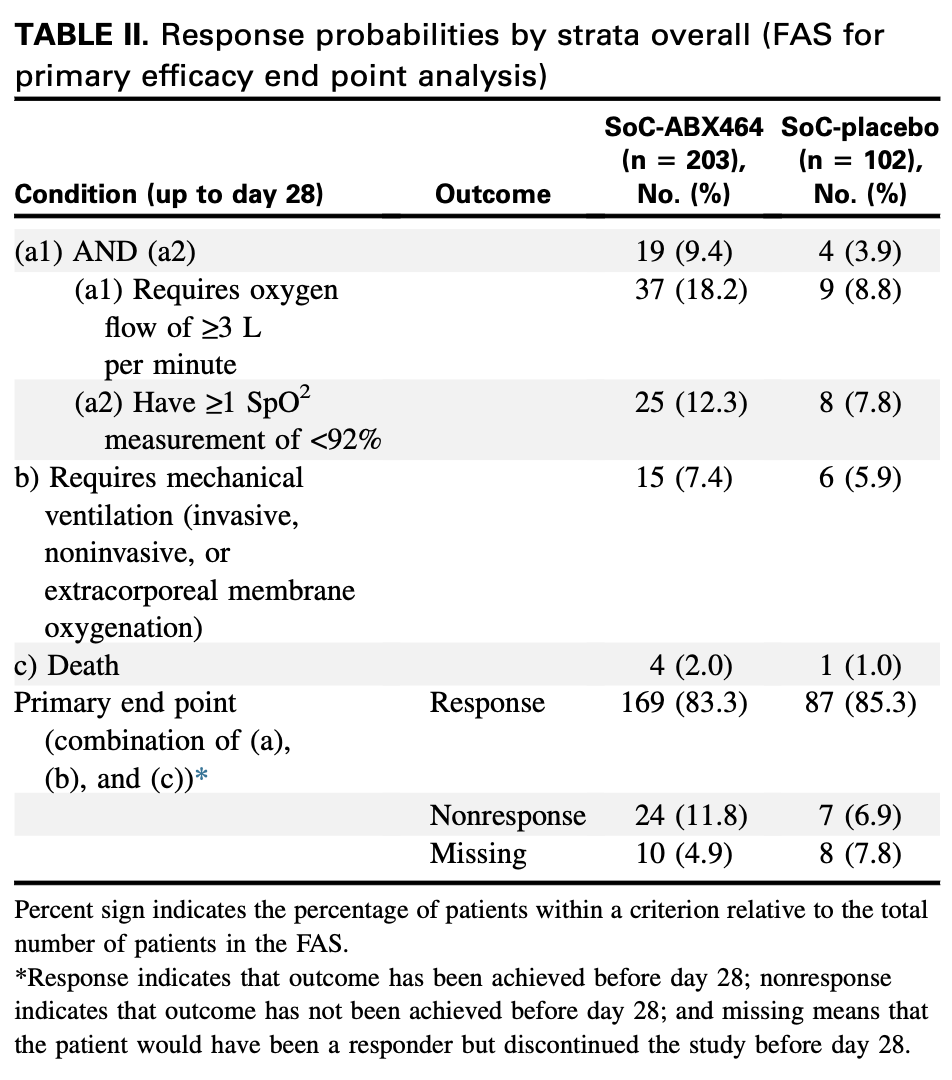
RCT 509 patients with COVID-19 at risk for severe disease showing no benefit with obefazimod (ABX464) 50mg daily for 28 days.
|
risk of death, 101.0% higher, RR 2.01, p = 0.67, treatment 4 of 203 (2.0%), control 1 of 102 (1.0%).
|
|
risk of mechanical ventilation, 25.6% higher, RR 1.26, p = 0.81, treatment 15 of 203 (7.4%), control 6 of 102 (5.9%).
|
|
risk of oxygen therapy, 138.7% higher, RR 2.39, p = 0.11, treatment 19 of 203 (9.4%), control 4 of 102 (3.9%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Giavina-Bianchi et al., 30 Nov 2023, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, 10 authors, average treatment delay 8.1 days, miR-AGE trial.
Contact: pbianchi@usp.br.
ABX464 (obefazimod) for patients with COVID-19 at risk for severe disease: miR-AGE, a randomized, double-blind placebo-controlled trial
Journal of Allergy and Clinical Immunology: Global, doi:10.1016/j.jacig.2023.100140
Background: ABX464 (obefazimod) is a small molecule that upregulates a single microRNA (miR-124) in immune cells and reduces the production of various inflammatory cytokines and chemokines. Objective: We assessed the efficacy and safety of the standard of care (SoC) plus oral obefazimod (SoC plus ABX464), 50 mg once daily, versus the SoC plus placebo for prevention of severe acute respiratory syndrome in patients with coronavirus disease 2019 (COVID-19) who are at risk for severe disease. Methods: Eligible patients for this phase 2/3 double-blind, placebo-controlled miR-AGE study were randomized (2:1) into 2 groups: SoC-ABX464 (n 5 339) and SoC-placebo (n 5 170). The primary end point was the percentage of patients who did not require use of high-flow oxygen or invasive or noninvasive mechanical ventilation within 28 days. The safety analyses included patients who had been randomly assigned and had received at least 1 dose of the study treatment. Results: At the time of the interim analysis, obefazimod showed no benefit over placebo when added to the SoC; the study enrollment was stopped for futility. The evaluation of the safety of obefazimod in 505 patients showed significantly more treatment-emergent adverse events in the SoC-ABX464 group than in the SoC-placebo group (P 5 .007). Frequently reported AEs in the SoC-ABX464 group included headache (14.6%), abdominal pain (9.6%), diarrhea (9.0%), back pain (6.9%), and nausea (6.0%). No treatment-related changes in laboratory parameters were reported. Conclusion: For patients who have severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and are at risk for severe COVID-19, obefazimod, 50 mg, provided no benefit over placebo when added to the SoC, although it did have a good safety profile (comparable to that reported in many therapeutic areas). (
References
Abivax, Investigator's brochure (7th ed) ABX464, Inflammatory bowel diseases
Apolit, Campos, Vautrin, Begon-Pescia, Lapasset et al., ABX464 (obefazimod) up-regulates miR-124 to reduce pro-inflammatory markers in inflammatory bowel diseases, Clin Transl Gastroenterol
Campos, Myburgh, Garcel, Vautrin, Lapasset et al., Long lasting control of viral rebound with a new drug ABX464 targeting Revmediated viral RNA biogenesis, Retrovirology
Chebli, Papon, Paul, Garcel, Campos et al., The anti-Hiv candidate Abx464 dampens intestinal inflammation by triggering Il-22 production in activated macrophages, Sci Rep
Daien, Krogulec, Gineste, Steens, Du Roure et al., Safety and efficacy of the miR-124 upregulator ABX464 (obefazimod, 50 and 100 mg per day) in patients with active rheumatoid arthritis and inadequate response to methotrexate and/or anti-TNFa therapy: a placebo-controlled phase II study, Ann Rheum Dis
Guo, Cao, Hong, Tan, Chen et al., The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak -an update on the status, Mil Med Res
Hoffmann, Kleine-Weber, Schroeder, Kr€ Uger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Kawano, Nakamachi, miR-124a as a key regulator of proliferation and MCP-1 secretion in synoviocytes from patients with rheumatoid arthritis, Ann Rheum Dis
Manchon, Chebli, Papon, Paul, Garcel et al., RNA sequencing analysis of activated macrophages treated with the anti-HIV ABX464 in intestinal inflammation, Sci Data
Qin, Wang, Su, Liu, miRNA-124 in immune system and immune disorders, Front Immunol
Rutsaert, Steens, Gineste, Cole, Kint et al., Safety, tolerability and impact on viral reservoirs of the addition to antiretroviral therapy of ABX464, an investigational antiviral drug, in individuals living with HIV-1: a phase IIa randomised controlled study, J Virus Erad
Scherrer, Rouzier, Barrett, Steens, Gineste et al., Pharmacokinetics and tolerability of ABX464, a novel first-in-class compound to treat HIV infection, in healthy HIV-uninfected subjects, J Antimicrob Chemother
Scherrer, Rouzier, Cardona, Barrett, Steens et al., Randomized trial of food effect on pharmacokinetic parameters of ABX464 administered orally to healthy male subjects, Antimicrob Agents Chemother
Steens, Scherrer, Gineste, Barrett, Khuanchai et al., Safety, pharmacokinetics, and antiviral activity of a novel HIV antiviral, ABX464, in treatment-naive HIV-infected subjects in a phase 2 randomized, controlled study, Antimicrob Agents Chemother
Sterne, Murthy, Diaz, Slutsky, Villar et al., Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis, JAMA
Tazi, Begon-Pescia, Campos, Apolit, Garcel et al., Specific and selective induction of miR-124 in immune cells by the quinoline ABX464: a transformative therapy for inflammatory diseases, Drug Discov Today
Vautrin, Manchon, Garcel, Campos, Lapasset et al., Both anti-inflammatory and antiviral properties of novel drug candidate ABX464 are mediated by modulation of RNA splicing, Sci Rep
Veremeyko, Siddiqui, Sotnikov, Yung, Ponomarev, IL-4/IL-13dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation, PLoS One
Vermeire, Hebuterne, Tilg, Hertogh, Gineste et al., Induction and long-term follow-up with ABX464 for moderate-to-severe ulcerative colitis: results of phase IIa trial, Gastroenterology
Vermeire, Sands, Tilg, Tulassay, Kempinski et al., ABX464 (obefazimod) for moderate to severe active ulcerative colitis: a randomised, placebo controlled phase 2b induction trial and 48 week extension, Lancet Gastroenterol Hepatol
DOI record:
{
"DOI": "10.1016/j.jacig.2023.100140",
"ISSN": [
"2772-8293"
],
"URL": "http://dx.doi.org/10.1016/j.jacig.2023.100140",
"alternative-id": [
"S2772829323000656"
],
"article-number": "100140",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "ABX464 (obefazimod) for patients with COVID-19 at risk for severe disease: miR-AGE, a randomized, double-blind placebo-controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Allergy and Clinical Immunology: Global"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jacig.2023.100140"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 Published by Elsevier Inc. on behalf of the American Academy of Allergy, Asthma & Immunology."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-1034-7580",
"affiliation": [],
"authenticated-orcid": false,
"family": "Giavina-Bianchi",
"given": "Pedro",
"sequence": "first"
},
{
"affiliation": [],
"family": "Cua",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Risso",
"given": "Karine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mondain",
"given": "Véronique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vissian",
"given": "Anaïs",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Joie",
"given": "Cécile",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pouletty",
"given": "Philippe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gineste",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ehrlich",
"given": "Hartmut J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kalil",
"given": "Jorge",
"sequence": "additional"
}
],
"container-title": "Journal of Allergy and Clinical Immunology: Global",
"container-title-short": "Journal of Allergy and Clinical Immunology: Global",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"jaci-global.org",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
7,
4
]
],
"date-time": "2023-07-04T21:29:08Z",
"timestamp": 1688506148000
},
"deposited": {
"date-parts": [
[
2025,
9,
9
]
],
"date-time": "2025-09-09T13:11:34Z",
"timestamp": 1757423494000
},
"indexed": {
"date-parts": [
[
2025,
9,
11
]
],
"date-time": "2025-09-11T21:00:59Z",
"timestamp": 1757624459075,
"version": "3.44.0"
},
"is-referenced-by-count": 2,
"issue": "4",
"issued": {
"date-parts": [
[
2023,
11
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2023,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T00:00:00Z",
"timestamp": 1698796800000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T00:00:00Z",
"timestamp": 1698796800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
30
]
],
"date-time": "2023-06-30T00:00:00Z",
"timestamp": 1688083200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2772829323000656?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2772829323000656?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100140",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
11
]
]
},
"published-print": {
"date-parts": [
[
2023,
11
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status",
"author": "Guo",
"first-page": "11",
"journal-title": "Mil Med Res",
"key": "10.1016/j.jacig.2023.100140_bib1",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "10.1016/j.jacig.2023.100140_bib2",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/s41598-018-37813-y",
"article-title": "Both anti-inflammatory and antiviral properties of novel drug candidate ABX464 are mediated by modulation of RNA splicing",
"author": "Vautrin",
"doi-asserted-by": "crossref",
"first-page": "792",
"journal-title": "Sci Rep",
"key": "10.1016/j.jacig.2023.100140_bib3",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1016/j.drudis.2020.12.019",
"article-title": "Specific and selective induction of miR-124 in immune cells by the quinoline ABX464: a transformative therapy for inflammatory diseases",
"author": "Tazi",
"doi-asserted-by": "crossref",
"first-page": "1030",
"journal-title": "Drug Discov Today",
"key": "10.1016/j.jacig.2023.100140_bib4",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1016/S2468-1253(22)00233-3",
"article-title": "ABX464 (obefazimod) for moderate to severe active ulcerative colitis: a randomised, placebo controlled phase 2b induction trial and 48 week extension",
"author": "Vermeire",
"doi-asserted-by": "crossref",
"first-page": "1024",
"journal-title": "Lancet Gastroenterol Hepatol",
"key": "10.1016/j.jacig.2023.100140_bib5",
"volume": "7",
"year": "2022"
},
{
"key": "10.1016/j.jacig.2023.100140_bib6",
"unstructured": "ABIVAX. Investigator's brochure (7th ed) ABX464. Inflammatory bowel diseases 2022. p 1-133."
},
{
"DOI": "10.14309/ctg.0000000000000560",
"article-title": "ABX464 (obefazimod) up-regulates miR-124 to reduce pro-inflammatory markers in inflammatory bowel diseases",
"author": "Apolit",
"doi-asserted-by": "crossref",
"journal-title": "Clin Transl Gastroenterol",
"key": "10.1016/j.jacig.2023.100140_bib7",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.3389/fimmu.2016.00406",
"article-title": "miRNA-124 in immune system and immune disorders",
"author": "Qin",
"doi-asserted-by": "crossref",
"first-page": "406",
"journal-title": "Front Immunol",
"key": "10.1016/j.jacig.2023.100140_bib8",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1186/s12977-015-0159-3",
"article-title": "Long lasting control of viral rebound with a new drug ABX464 targeting Rev-mediated viral RNA biogenesis",
"author": "Campos",
"doi-asserted-by": "crossref",
"first-page": "30",
"journal-title": "Retrovirology",
"key": "10.1016/j.jacig.2023.100140_bib9",
"volume": "12",
"year": "2015"
},
{
"article-title": "Pharmacokinetics and tolerability of ABX464, a novel first-in-class compound to treat HIV infection, in healthy HIV-uninfected subjects",
"author": "Scherrer",
"first-page": "820",
"journal-title": "J Antimicrob Chemother",
"key": "10.1016/j.jacig.2023.100140_bib10",
"volume": "72",
"year": "2017"
},
{
"DOI": "10.1128/AAC.00545-17",
"article-title": "Safety, pharmacokinetics, and antiviral activity of a novel HIV antiviral, ABX464, in treatment-naive HIV-infected subjects in a phase 2 randomized, controlled study",
"author": "Steens",
"doi-asserted-by": "crossref",
"first-page": "e00545",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.jacig.2023.100140_bib11",
"volume": "61",
"year": "2017"
},
{
"DOI": "10.1016/S2055-6640(20)30273-9",
"article-title": "Safety, tolerability and impact on viral reservoirs of the addition to antiretroviral therapy of ABX464, an investigational antiviral drug, in individuals living with HIV-1: a phase IIa randomised controlled study",
"author": "Rutsaert",
"doi-asserted-by": "crossref",
"first-page": "10",
"journal-title": "J Virus Erad",
"key": "10.1016/j.jacig.2023.100140_bib12",
"volume": "5",
"year": "2019"
},
{
"DOI": "10.1136/ard.2010.138669",
"article-title": "miR-124a as a key regulator of proliferation and MCP-1 secretion in synoviocytes from patients with rheumatoid arthritis",
"author": "Kawano",
"doi-asserted-by": "crossref",
"first-page": "i88",
"issue": "suppl 1",
"journal-title": "Ann Rheum Dis",
"key": "10.1016/j.jacig.2023.100140_bib13",
"volume": "70",
"year": "2011"
},
{
"DOI": "10.1371/journal.pone.0081774",
"article-title": "IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation",
"author": "Veremeyko",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/j.jacig.2023.100140_bib14",
"volume": "8",
"year": "2013"
},
{
"DOI": "10.1038/s41598-017-04071-3",
"article-title": "The anti-Hiv candidate Abx464 dampens intestinal inflammation by triggering Il-22 production in activated macrophages",
"author": "Chebli",
"doi-asserted-by": "crossref",
"first-page": "4860",
"journal-title": "Sci Rep",
"key": "10.1016/j.jacig.2023.100140_bib15",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1038/sdata.2017.150",
"article-title": "RNA sequencing analysis of activated macrophages treated with the anti-HIV ABX464 in intestinal inflammation",
"author": "Manchon",
"doi-asserted-by": "crossref",
"journal-title": "Sci Data",
"key": "10.1016/j.jacig.2023.100140_bib16",
"volume": "4",
"year": "2017"
},
{
"DOI": "10.1001/jama.2020.17023",
"article-title": "Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis",
"author": "Sterne",
"doi-asserted-by": "crossref",
"first-page": "1330",
"journal-title": "JAMA",
"key": "10.1016/j.jacig.2023.100140_bib17",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1128/AAC.01288-16",
"article-title": "Randomized trial of food effect on pharmacokinetic parameters of ABX464 administered orally to healthy male subjects",
"author": "Scherrer",
"doi-asserted-by": "crossref",
"first-page": "e01288",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.jacig.2023.100140_bib18",
"volume": "61",
"year": "2017"
},
{
"DOI": "10.1053/j.gastro.2021.02.054",
"article-title": "Induction and long-term follow-up with ABX464 for moderate-to-severe ulcerative colitis: results of phase IIa trial",
"author": "Vermeire",
"doi-asserted-by": "crossref",
"first-page": "2595",
"journal-title": "Gastroenterology",
"key": "10.1016/j.jacig.2023.100140_bib19",
"volume": "160",
"year": "2021"
},
{
"DOI": "10.1136/annrheumdis-2022-222228",
"article-title": "Safety and efficacy of the miR-124 upregulator ABX464 (obefazimod, 50 and 100 mg per day) in patients with active rheumatoid arthritis and inadequate response to methotrexate and/or anti-TNFα therapy: a placebo-controlled phase II study",
"author": "Daien",
"doi-asserted-by": "crossref",
"first-page": "1076",
"journal-title": "Ann Rheum Dis",
"key": "10.1016/j.jacig.2023.100140_bib20",
"volume": "81",
"year": "2022"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2772829323000656"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "ABX464 (obefazimod) for patients with COVID-19 at risk for severe disease: miR-AGE, a randomized, double-blind placebo-controlled trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "2"
}