Statin and aspirin as adjuvant therapy in hospitalised patients with SARS-CoV-2 infection: a randomised clinical trial (RESIST trial)
et al., BMC Infectious Diseases, doi:10.1186/s12879-022-07570-5, RESIST, CTRI/2020/07/026791, Jul 2022
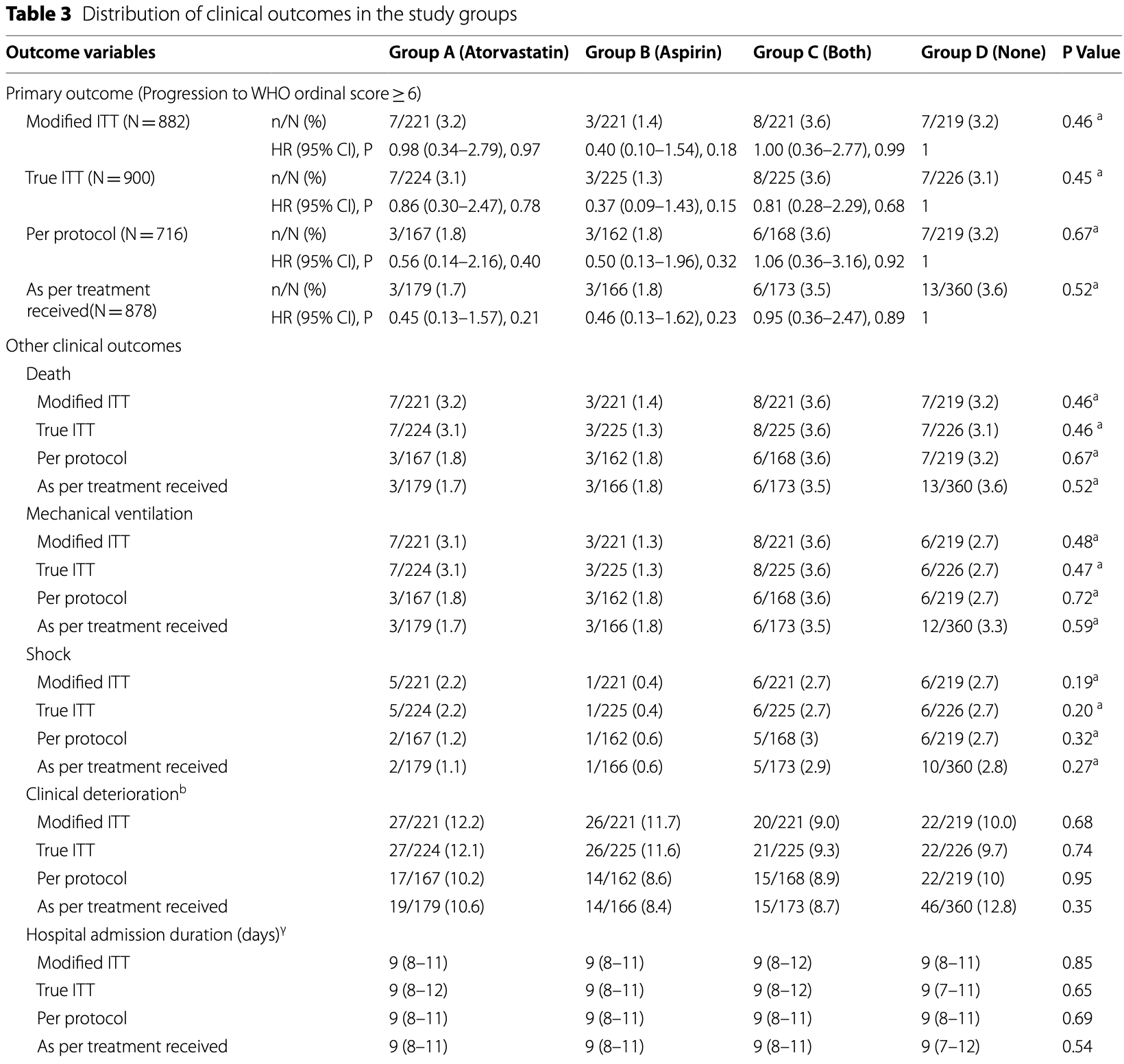
RCT hospitalized patients in India, 224 treated with atorvastatin, 225 with aspirin, and 225 with both, showing lower serum interleukin-6 levels with aspirin, but no statistically significant changes in other outcomes. Low dose aspirin 75mg daily for 10 days.
|
risk of death, 22.1% lower, RR 0.78, p = 0.62, treatment 11 of 442 (2.5%), control 7 of 219 (3.2%), NNT 141, aspirin and aspirin/atorvastatin vs. control, modified intention-to-treat.
|
|
risk of death, 57.5% lower, RR 0.42, p = 0.22, treatment 3 of 221 (1.4%), control 7 of 219 (3.2%), NNT 54, aspirin vs. control, modified intention-to-treat.
|
|
risk of mechanical ventilation, 9.2% lower, RR 0.91, p = 0.80, treatment 11 of 442 (2.5%), control 6 of 219 (2.7%), NNT 398, aspirin and aspirin/atorvastatin vs. control, modified intention-to-treat.
|
|
risk of mechanical ventilation, 50.5% lower, RR 0.50, p = 0.34, treatment 3 of 221 (1.4%), control 6 of 219 (2.7%), NNT 72, aspirin vs. control, modified intention-to-treat.
|
|
risk of progression, 30.0% lower, HR 0.70, p = 0.46, treatment 11 of 442 (2.5%), control 7 of 219 (3.2%), NNT 141, aspirin and aspirin/atorvastatin vs. control, Cox proportional hazards, modified intention-to-treat, primary outcome.
|
|
risk of progression, 60.0% lower, HR 0.40, p = 0.18, treatment 3 of 221 (1.4%), control 7 of 219 (3.2%), NNT 54, aspirin vs. control, Cox proportional hazards, modified intention-to-treat, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ghati et al., 9 Jul 2022, Randomized Controlled Trial, India, peer-reviewed, 14 authors, study period 28 July, 2020 - 27 January, 2021, average treatment delay 6.0 days, trial CTRI/2020/07/026791 (RESIST).
Contact: deeptikailath@gmail.com (corresponding author).
Statin and aspirin as adjuvant therapy in hospitalised patients with SARS-CoV-2 infection: a randomised clinical trial (RESIST trial)
BMC Infectious Diseases, doi:10.1186/s12879-022-07570-5
Background: Statins and aspirin have been proposed for treatment of COVID-19 because of their anti-inflammatory and anti-thrombotic properties. Several observational studies have shown favourable results. There is a need for a randomised controlled trial.
Methods: In this single-center, open-label, randomised controlled trial, 900 RT-PCR positive COVID-19 patients requiring hospitalisation, were randomly assigned to receive either atorvastatin 40 mg (Group A, n = 224), aspirin 75 mg (Group B, n = 225), or both (Group C, n = 225) in addition to standard of care for 10 days or until discharge whichever was earlier or only standard of care (Group D, n = 226). The primary outcome variable was clinical deterioration to WHO Ordinal Scale for Clinical Improvement ≥ 6. The secondary outcome was change in serum C-reactive protein, interleukin-6, and troponin I.
Results: The primary outcome occurred in 25 (2.8%) patients: 7 (3.2%) in Group A, 3 (1.4%) in Group B, 8 (3.6%) in Group C, and 7 (3.2%) in Group D. There was no difference in primary outcome across the study groups (P = 0.463). Comparison of all patients who received atorvastatin or aspirin with the control group (Group D) also did not show any benefit [Atorvastatin: HR 1.0 (95% CI 0.41-2.46) P = 0.99; Aspirin: HR 0.7 (95% CI 0.27-1.81) P = 0.46]. The secondary outcomes revealed lower serum interleukin-6 levels among patients in Groups B and C. There was no excess of adverse events. Conclusions: Among patients admitted with mild to moderate COVID-19 infection, additional treatment with aspirin, atorvastatin, or a combination of the two does not prevent clinical deterioration.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s12879-022-07570-5. Additional file 1. eFigure 1. Institute Covid-19 treatment protocol. eFigure 2. Probability of having WHO Ordinal Scale for Clinical Improvement < 6 in the study groups over time (Intension-to-treat analysis). eFigure 3. Probability of having WHO Ordinal Scale for Clinical Improvement < 6 in the study groups over time (Per protocol analysis). eFigure 4. Probability of having WHO Ordinal Scale for Clinical Improvement < 6 in the study groups over time (as treated analysis). eTable 1. Distribution of adverse events in the study groups. Author contributions NG: Conceptualization, Methodology, Data curation, Visualization, Writing-Original draft preparation, Writing-Review and Editing; SuB: Project Administration, Supervision, Resource; MM, AT,KP, DK: Data curation, Methodology, Investigation; PT, TD: Methodology, Investigation; KM: Software, Formal Analysis, Validation; RiG, AM, RG, AS: Supervision, Resources; SD: Project Administration, Supervision, Conceptualization, Methodology, Investigation, Data curation, Visualization, Writing-Original draft preparation, Writing-Review and Editing. NG, SD have verified the underlying data. All authors have read and approved the final manuscript.
Funding There was no funding source for this study.
Declarations Ethics approval and consent to participate The trial was conducted in accordance..
References
Almog, Shefer, Novack, Maimon, Barski et al., Prior statin therapy is associated with a decreased rate of severe sepsis, Circulation
Castiglione, Chiriacò, Emdin, Taddei, Vergaro, Statin therapy in COVID-19 infection, Eur Heart J Cardiovasc Pharmacother
Cheruiyot, Kipkorir, Ngure, Misiani, Munguti et al., Arterial thrombosis in coronavirus disease 2019 patients: a rapid systematic review, Ann Vasc Surg
Chow, Khanna, Kethireddy, Yamane, Levine et al., Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019, Anesth Analg
Dobesh, Klepser, Mcguire, Morgan, Olsen, Reduction in mortality associated with statin therapy in patients with severe sepsis, Pharmacotherapy
Fedson, Opal, Rordam, Hiding in plain sight: an approach to treating patients with severe COVID-19 infection, mBio
Ghati, Roy, Bhatnagar, Bhati, Bhushan et al., Atorvastatin and Aspirin as Adjuvant Therapy in Patients with SARS-CoV-2 Infection: a structured summary of a study protocol for a randomised controlled trial, Trials
Glatthaar-Saalmüller, Mair, Saalmüller, Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study, Influenza Other Respir Viruses
Hariyanto, Kurniawan, Statin therapy did not improve the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection, Diabetes Metab Syndr
Iwata, Shirai, Ishii, Kushima, Otani et al., Inhibitory effect of statins on inflammatory cytokine production from human bronchial epithelial cells, Clin Exp Immunol
Kor, Carter, Park, Festic, Banner-Goodspeed et al., Effect of aspirin on development of ARDS in at-risk patients presenting to the emergency department: the LIPS-a randomized clinical trial, JAMA
Kow, Hasan, Meta-analysis of effect of statins in patients with COVID-19, Am J Cardiol
Madjid, Safavi-Naeini, Solomon, Vardeny, Potential effects of coronaviruses on the cardiovascular system: a review, JAMA Cardiol
Makris, Manoulakas, Komnos, Papakrivou, Tzovaras et al., Effect of pravastatin on the frequency of ventilator-associated pneumonia and on intensive care unit mortality: open-label, randomized study, Crit Care Med
Mazur, Wurzer, Ehrhardt, Pleschka, Puthavathana et al., Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF-kappaB-inhibiting activity, Cell Microbiol
Melo, Valença, Gitirana, Santos, Ribeiro et al., Redox markers and inflammation are differentially affected by atorvastatin, pravastatin or simvastatin administered before endotoxin-induced acute lung injury, Int Immunopharmacol
Morris, Stables, Hobbs, De Souza, Colville-Nash et al., Effects of low-dose aspirin on acute inflammatory responses in humans, J Immunol
Onorato, Pucci, Carpene, Henry, Sanchis-Gomar et al., Protective effects of statins administration in European and north American patients infected with COVID-19: a meta-analysis, Semin Thromb Hemost
Osborne, Veigulis, Arreola, Mahajan, Röösli et al., Association of mortality and aspirin prescription for COVID-19 patients at the Veterans Health Administration, PLoS ONE
Pal, Banerjee, Yadav, Bhattacharjee, Statin use and clinical outcomes in patients with COVID-19: an updated systematic review and meta-analysis, Postgrad Med J
Papazian, Roch, Charles, Penot-Ragon, Perrin et al., Effect of statin therapy on mortality in patients with ventilator-associated pneumonia: a randomized clinical trial, JAMA
Recovery Collaborative, Horby, Pessoa-Amorim, Staplin, Emberson et al., Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
Scheen, Statins and clinical outcomes with COVID-19: meta-analyses of observational studies, Diabetes Metab
Somasundaram, Sigthorsson, Simpson, Watts, Jacob et al., Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAIDenteropathy in the rat, Aliment Pharmacol Ther
Totura, Whitmore, Agnihothram, Schäfer, Katze et al., Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection, MBio
Van Cao, Moradi-Bidhendi, Cooper, Gilroy, 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation, J Exp Med
Viecca, Radovanovic, Forleo, Santus, Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study, Pharmacol Res
Wichmann, Sperhake, Lütgehetmann, Steurer, Edler et al., Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study, Ann Intern Med
Yuan, Chen, Li, Chen, Wang et al., Mortality and pre-hospitalization use of low-dose aspirin in COVID-19 patients with coronary artery disease, J Cell Mol Med
Yuan, Deng, Guo, Shang, Zhu et al., Atorvastatin attenuates myocardial remodeling induced by chronic intermittent hypoxia in rats: partly involvement of TLR-4/MYD88 pathway, Biochem Biophys Res Commun
DOI record:
{
"DOI": "10.1186/s12879-022-07570-5",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-022-07570-5",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Statins and aspirin have been proposed for treatment of COVID-19 because of their anti-inflammatory and anti-thrombotic properties. Several observational studies have shown favourable results. There is a need for a randomised controlled trial.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>In this single-center, open-label, randomised controlled trial, 900 RT-PCR positive COVID-19 patients requiring hospitalisation, were randomly assigned to receive either atorvastatin 40 mg (Group A, n = 224), aspirin 75 mg (Group B, n = 225), or both (Group C, n = 225) in addition to standard of care for 10 days or until discharge whichever was earlier or only standard of care (Group D, n = 226). The primary outcome variable was clinical deterioration to WHO Ordinal Scale for Clinical Improvement ≥ 6. The secondary outcome was change in serum C-reactive protein, interleukin-6, and troponin I.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>The primary outcome occurred in 25 (2.8%) patients: 7 (3.2%) in Group A, 3 (1.4%) in Group B, 8 (3.6%) in Group C, and 7 (3.2%) in Group D. There was no difference in primary outcome across the study groups (P = 0.463). Comparison of all patients who received atorvastatin or aspirin with the control group (Group D) also did not show any benefit [Atorvastatin: HR 1.0 (95% CI 0.41–2.46) P = 0.99; Aspirin: HR 0.7 (95% CI 0.27–1.81) P = 0.46]. The secondary outcomes revealed lower serum interleukin-6 levels among patients in Groups B and C. There was no excess of adverse events.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Among patients admitted with mild to moderate COVID-19 infection, additional treatment with aspirin, atorvastatin, or a combination of the two does not prevent clinical deterioration.</jats:p>\n <jats:p><jats:italic>Trial Registry Number</jats:italic> CTRI/2020/07/026791 (<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"http://ctri.nic.in\">http://ctri.nic.in</jats:ext-link>; registered on 25/07/2020)</jats:p>\n </jats:sec>",
"alternative-id": [
"7570"
],
"article-number": "606",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "8 March 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "27 May 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "9 July 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The trial was conducted in accordance with the 1964 Helsinki Declaration and its later amendments. The trial was approved by the institutional ethical committee (attached). Informed consent was obtained from all participants."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "We declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Ghati",
"given": "Nirmal",
"sequence": "first"
},
{
"affiliation": [],
"family": "Bhatnagar",
"given": "Sushma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahendran",
"given": "Manjit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thakur",
"given": "Abhishek",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prasad",
"given": "Kshitij",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Devesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dwivedi",
"given": "Tanima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mani",
"given": "Kalaivani",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tiwari",
"given": "Pawan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gupta",
"given": "Ritu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mohan",
"given": "Anant",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saxena",
"given": "Anita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guleria",
"given": "Randeep",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deepti",
"given": "Siddharthan",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
7,
9
]
],
"date-time": "2022-07-09T07:02:59Z",
"timestamp": 1657350179000
},
"deposited": {
"date-parts": [
[
2022,
7,
9
]
],
"date-time": "2022-07-09T07:03:24Z",
"timestamp": 1657350204000
},
"indexed": {
"date-parts": [
[
2022,
7,
10
]
],
"date-time": "2022-07-10T12:41:02Z",
"timestamp": 1657456862818
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
7,
9
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
9
]
],
"date-time": "2022-07-09T00:00:00Z",
"timestamp": 1657324800000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
9
]
],
"date-time": "2022-07-09T00:00:00Z",
"timestamp": 1657324800000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-022-07570-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-022-07570-5/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-022-07570-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2022,
7,
9
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
9
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1093/ehjcvp/pvaa042",
"author": "V Castiglione",
"doi-asserted-by": "publisher",
"first-page": "258",
"issue": "4",
"journal-title": "Eur Heart J Cardiovasc Pharmacother",
"key": "7570_CR1",
"unstructured": "Castiglione V, Chiriacò M, Emdin M, Taddei S, Vergaro G. Statin therapy in COVID-19 infection. Eur Heart J Cardiovasc Pharmacother. 2020;6(4):258–9.",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1097/CCM.0b013e318225742c",
"author": "D Makris",
"doi-asserted-by": "publisher",
"first-page": "2440",
"issue": "11",
"journal-title": "Crit Care Med",
"key": "7570_CR2",
"unstructured": "Makris D, Manoulakas E, Komnos A, Papakrivou E, Tzovaras N, Hovas A, Zintzaras E, Zakynthinos E. Effect of pravastatin on the frequency of ventilator-associated pneumonia and on intensive care unit mortality: open-label, randomized study. Crit Care Med. 2011;39(11):2440–6.",
"volume": "39",
"year": "2011"
},
{
"DOI": "10.1001/jama.2013.280031",
"author": "L Papazian",
"doi-asserted-by": "publisher",
"first-page": "1692",
"issue": "16",
"journal-title": "JAMA",
"key": "7570_CR3",
"unstructured": "Papazian L, Roch A, Charles PE, Penot-Ragon C, Perrin G, Roulier P, Goutorbe P, Lefrant JY, Wiramus S, Jung B, Perbet S, Hernu R, Nau A, Baldesi O, Allardet-Servent J, Baumstarck K, Jouve E, Moussa M, Hraiech S, Guervilly C, Forel JM. Effect of statin therapy on mortality in patients with ventilator-associated pneumonia: a randomized clinical trial. JAMA. 2013;310(16):1692–700.",
"volume": "310",
"year": "2013"
},
{
"DOI": "10.1161/01.CIR.0000138932.17956.F1",
"author": "Y Almog",
"doi-asserted-by": "publisher",
"first-page": "880",
"issue": "7",
"journal-title": "Circulation",
"key": "7570_CR4",
"unstructured": "Almog Y, Shefer A, Novack V, Maimon N, Barski L, Eizinger M, Friger M, Zeller L, Danon A. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110(7):880–5.",
"volume": "110",
"year": "2004"
},
{
"DOI": "10.1592/phco.29.6.621",
"author": "PP Dobesh",
"doi-asserted-by": "publisher",
"first-page": "621",
"issue": "6",
"journal-title": "Pharmacotherapy",
"key": "7570_CR5",
"unstructured": "Dobesh PP, Klepser DG, McGuire TR, Morgan CW, Olsen KM. Reduction in mortality associated with statin therapy in patients with severe sepsis. Pharmacotherapy. 2009;29(6):621–30.",
"volume": "29",
"year": "2009"
},
{
"DOI": "10.1016/j.amjcard.2020.08.004",
"author": "CS Kow",
"doi-asserted-by": "publisher",
"first-page": "153",
"journal-title": "Am J Cardiol",
"key": "7570_CR6",
"unstructured": "Kow CS, Hasan SS. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 2020;134:153–5.",
"volume": "134",
"year": "2020"
},
{
"DOI": "10.1055/s-0040-1722307",
"author": "D Onorato",
"doi-asserted-by": "publisher",
"first-page": "392",
"issue": "4",
"journal-title": "Semin Thromb Hemost",
"key": "7570_CR7",
"unstructured": "Onorato D, Pucci M, Carpene G, Henry BM, Sanchis-Gomar F, Lippi G. Protective effects of statins administration in European and north American patients infected with COVID-19: a meta-analysis. Semin Thromb Hemost. 2021;47(4):392–9.",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1136/postgradmedj-2020-139172",
"author": "R Pal",
"doi-asserted-by": "publisher",
"first-page": "354",
"journal-title": "Postgrad Med J",
"key": "7570_CR8",
"unstructured": "Pal R, Banerjee M, Yadav U, Bhattacharjee S. Statin use and clinical outcomes in patients with COVID-19: an updated systematic review and meta-analysis. Postgrad Med J. 2021;98:354.",
"volume": "98",
"year": "2021"
},
{
"DOI": "10.1016/j.dsx.2020.08.023",
"author": "TI Hariyanto",
"doi-asserted-by": "publisher",
"first-page": "1613",
"issue": "6",
"journal-title": "Diabetes Metab Syndr",
"key": "7570_CR9",
"unstructured": "Hariyanto TI, Kurniawan A. Statin therapy did not improve the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(6):1613–5.",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.diabet.2020.101220",
"author": "AJ Scheen",
"doi-asserted-by": "publisher",
"issue": "6",
"journal-title": "Diabetes Metab",
"key": "7570_CR10",
"unstructured": "Scheen AJ. Statins and clinical outcomes with COVID-19: meta-analyses of observational studies. Diabetes Metab. 2020;47(6): 101220.",
"volume": "47",
"year": "2020"
},
{
"DOI": "10.4049/jimmunol.0900477",
"author": "T Morris",
"doi-asserted-by": "publisher",
"first-page": "2089",
"issue": "3",
"journal-title": "J Immunol",
"key": "7570_CR11",
"unstructured": "Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, Warner T, Newson J, Bellingan G, Gilroy DW. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183(3):2089–96.",
"volume": "183",
"year": "2009"
},
{
"DOI": "10.1001/jama.2016.6330",
"author": "DJ Kor",
"doi-asserted-by": "publisher",
"first-page": "2406",
"issue": "22",
"journal-title": "JAMA",
"key": "7570_CR12",
"unstructured": "Kor DJ, Carter RE, Park PK, Festic E, Banner-Goodspeed VM, Hinds R, Talmor D, Gajic O, Ware LB, Gong MN. Effect of aspirin on development of ARDS in at-risk patients presenting to the emergency department: the LIPS-a randomized clinical trial. JAMA. 2016;315(22):2406–14.",
"volume": "315",
"year": "2016"
},
{
"DOI": "10.1111/irv.12421",
"author": "B Glatthaar-Saalmüller",
"doi-asserted-by": "publisher",
"first-page": "85",
"issue": "1",
"journal-title": "Influenza Other Respir Viruses",
"key": "7570_CR13",
"unstructured": "Glatthaar-Saalmüller B, Mair KH, Saalmüller A. Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study. Influenza Other Respir Viruses. 2017;11(1):85–92.",
"volume": "11",
"year": "2017"
},
{
"DOI": "10.1016/j.phrs.2020.104950",
"author": "M Viecca",
"doi-asserted-by": "publisher",
"first-page": "104950",
"journal-title": "Pharmacol Res",
"key": "7570_CR14",
"unstructured": "Viecca M, Radovanovic D, Forleo GB, Santus P. Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study. Pharmacol Res. 2020;158:104950.",
"volume": "158",
"year": "2020"
},
{
"DOI": "10.1213/ANE.0000000000005292",
"author": "JH Chow",
"doi-asserted-by": "publisher",
"first-page": "930",
"issue": "4",
"journal-title": "Anesth Analg",
"key": "7570_CR15",
"unstructured": "Chow JH, Khanna AK, Kethireddy S, Yamane D, Levine A, Jackson AM, McCurdy MT, Tabatabai A, Kumar G, Park P, Benjenk I, Menaker J, Ahmed N, Glidewell E, Presutto E, Cain S, Haridasa N, Field W, Fowler JG, Trinh D, Johnson KN, Kaur A, Lee A, Sebastian K, Ulrich A, Peña S, Carpenter R, Sudhakar S, Uppal P, Fedeles BT, Sachs A, Dahbour L, Teeter W, Tanaka K, Galvagno SM, Herr DL, Scalea TM, Mazzeffi MA. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth Analg. 2021;132(4):930–41.",
"volume": "132",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0246825",
"author": "TF Osborne",
"doi-asserted-by": "publisher",
"issue": "2",
"journal-title": "PLoS ONE",
"key": "7570_CR16",
"unstructured": "Osborne TF, Veigulis ZP, Arreola DM, Mahajan SM, Röösli E, Curtin CM. Association of mortality and aspirin prescription for COVID-19 patients at the Veterans Health Administration. PLoS ONE. 2021;16(2): e0246825.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1111/jcmm.16198",
"author": "S Yuan",
"doi-asserted-by": "publisher",
"first-page": "1263",
"issue": "2",
"journal-title": "J Cell Mol Med",
"key": "7570_CR17",
"unstructured": "Yuan S, Chen P, Li H, Chen C, Wang F, Wang DW. Mortality and pre-hospitalization use of low-dose aspirin in COVID-19 patients with coronary artery disease. J Cell Mol Med. 2021;25(2):1263–73.",
"volume": "25",
"year": "2021"
},
{
"key": "7570_CR18",
"unstructured": "RECOVERY Collaborative Group; Horby PW, Pessoa-Amorim G, Staplin N, Emberson JR, Campbell M, Spata E, Peto L, Brunskill NJ, Tiberi S, Chew V, Brown T, Tahir H, Ebert B, Chadwick D, Whitehouse T, Sarkar R, Graham C, Baillie JK, Basnyat B, Buch MH, Chappell LC, Day J, Faust SN, Hamers RL, Jaki T, Juszczak E, Jeffery K, Lim WS, Montgomery A, Mumford A, Rowan K, Thwaites G, Mafham M, Haynes R, Landray MJ. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv 2021:2021.06.08.21258132."
},
{
"DOI": "10.1186/s13063-020-04840-y",
"author": "N Ghati",
"doi-asserted-by": "publisher",
"first-page": "902",
"issue": "1",
"journal-title": "Trials",
"key": "7570_CR19",
"unstructured": "Ghati N, Roy A, Bhatnagar S, Bhati S, Bhushan S, Mahendran M, Thakur A, Tiwari P, Dwivedi T, Mani K, Gupta R, Mohan A, Garg R, Saxena A, Guleria R, Deepti S. Atorvastatin and Aspirin as Adjuvant Therapy in Patients with SARS-CoV-2 Infection: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):902.",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1111/j.1365-2249.2012.04564.x",
"author": "A Iwata",
"doi-asserted-by": "publisher",
"first-page": "234",
"issue": "2",
"journal-title": "Clin Exp Immunol",
"key": "7570_CR20",
"unstructured": "Iwata A, Shirai R, Ishii H, Kushima H, Otani S, Hashinaga K, Umeki K, Kishi K, Tokimatsu I, Hiramatsu K, Kadota J. Inhibitory effect of statins on inflammatory cytokine production from human bronchial epithelial cells. Clin Exp Immunol. 2012;168(2):234–40.",
"volume": "168",
"year": "2012"
},
{
"DOI": "10.1016/j.intimp.2013.05.016",
"author": "AC Melo",
"doi-asserted-by": "publisher",
"first-page": "57",
"issue": "1",
"journal-title": "Int Immunopharmacol",
"key": "7570_CR21",
"unstructured": "Melo AC, Valença SS, Gitirana LB, Santos JC, Ribeiro ML, Machado MN, Magalhães CB, Zin WA, Porto LC. Redox markers and inflammation are differentially affected by atorvastatin, pravastatin or simvastatin administered before endotoxin-induced acute lung injury. Int Immunopharmacol. 2013;17(1):57–64.",
"volume": "17",
"year": "2013"
},
{
"DOI": "10.1128/mBio.00638-15",
"author": "AL Totura",
"doi-asserted-by": "publisher",
"first-page": "e00638",
"issue": "3",
"journal-title": "MBio",
"key": "7570_CR22",
"unstructured": "Totura AL, Whitmore A, Agnihothram S, Schäfer A, Katze MG, Heise MT, Baric RS. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. MBio. 2015;6(3):e00638-e715.",
"volume": "6",
"year": "2015"
},
{
"DOI": "10.1016/j.bbrc.2014.02.091",
"author": "X Yuan",
"doi-asserted-by": "publisher",
"first-page": "292",
"issue": "1",
"journal-title": "Biochem Biophys Res Commun",
"key": "7570_CR23",
"unstructured": "Yuan X, Deng Y, Guo X, Shang J, Zhu D, Liu H. Atorvastatin attenuates myocardial remodeling induced by chronic intermittent hypoxia in rats: partly involvement of TLR-4/MYD88 pathway. Biochem Biophys Res Commun. 2014;446(1):292–7.",
"volume": "446",
"year": "2014"
},
{
"DOI": "10.1001/jamacardio.2020.1286",
"author": "M Madjid",
"doi-asserted-by": "publisher",
"first-page": "831",
"issue": "7",
"journal-title": "JAMA Cardiol",
"key": "7570_CR24",
"unstructured": "Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–40.",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1128/mBio.00398-20",
"doi-asserted-by": "crossref",
"key": "7570_CR25",
"unstructured": "Fedson DS, Opal SM, Rordam OM. Hiding in plain sight: an approach to treating patients with severe COVID-19 infection. mBio 2020;11(2)."
},
{
"DOI": "10.7326/M20-2003",
"author": "D Wichmann",
"doi-asserted-by": "publisher",
"first-page": "268",
"issue": "4",
"journal-title": "Ann Intern Med",
"key": "7570_CR26",
"unstructured": "Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–77.",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1016/j.avsg.2020.08.087",
"author": "I Cheruiyot",
"doi-asserted-by": "publisher",
"first-page": "273",
"journal-title": "Ann Vasc Surg",
"key": "7570_CR27",
"unstructured": "Cheruiyot I, Kipkorir V, Ngure B, Misiani M, Munguti J, Ogeng’o J. Arterial thrombosis in coronavirus disease 2019 patients: a rapid systematic review. Ann Vasc Surg. 2021;70:273–81.",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1046/j.1365-2036.2000.00723.x",
"author": "S Somasundaram",
"doi-asserted-by": "publisher",
"first-page": "639",
"issue": "5",
"journal-title": "Aliment Pharmacol Ther",
"key": "7570_CR28",
"unstructured": "Somasundaram S, Sigthorsson G, Simpson RJ, Watts J, Jacob M, Tavares IA, Rafi S, Roseth A, Foster R, Price AB, Wrigglesworth JM, Bjarnason I. Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat. Aliment Pharmacol Ther. 2000;14(5):639–50.",
"volume": "14",
"year": "2000"
},
{
"DOI": "10.1084/jem.20040566",
"author": "MJ Paul-Clark",
"doi-asserted-by": "publisher",
"first-page": "69",
"issue": "1",
"journal-title": "J Exp Med",
"key": "7570_CR29",
"unstructured": "Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200(1):69–78.",
"volume": "200",
"year": "2004"
},
{
"DOI": "10.1111/j.1462-5822.2007.00902.x",
"author": "I Mazur",
"doi-asserted-by": "publisher",
"first-page": "1683",
"issue": "7",
"journal-title": "Cell Microbiol",
"key": "7570_CR30",
"unstructured": "Mazur I, Wurzer WJ, Ehrhardt C, Pleschka S, Puthavathana P, Silberzahn T, Wolff T, Planz O, Ludwig S. Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF-kappaB-inhibiting activity. Cell Microbiol. 2007;9(7):1683–94.",
"volume": "9",
"year": "2007"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-022-07570-5"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases"
],
"subtitle": [],
"title": "Statin and aspirin as adjuvant therapy in hospitalised patients with SARS-CoV-2 infection: a randomised clinical trial (RESIST trial)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "22"
}