Clinical effects of dexamethasone among patients with sickle cell disease hospitalized with COVID-19: Outcomes from a single academic health system
et al., PLOS ONE, doi:10.1371/journal.pone.0313289, Nov 2024
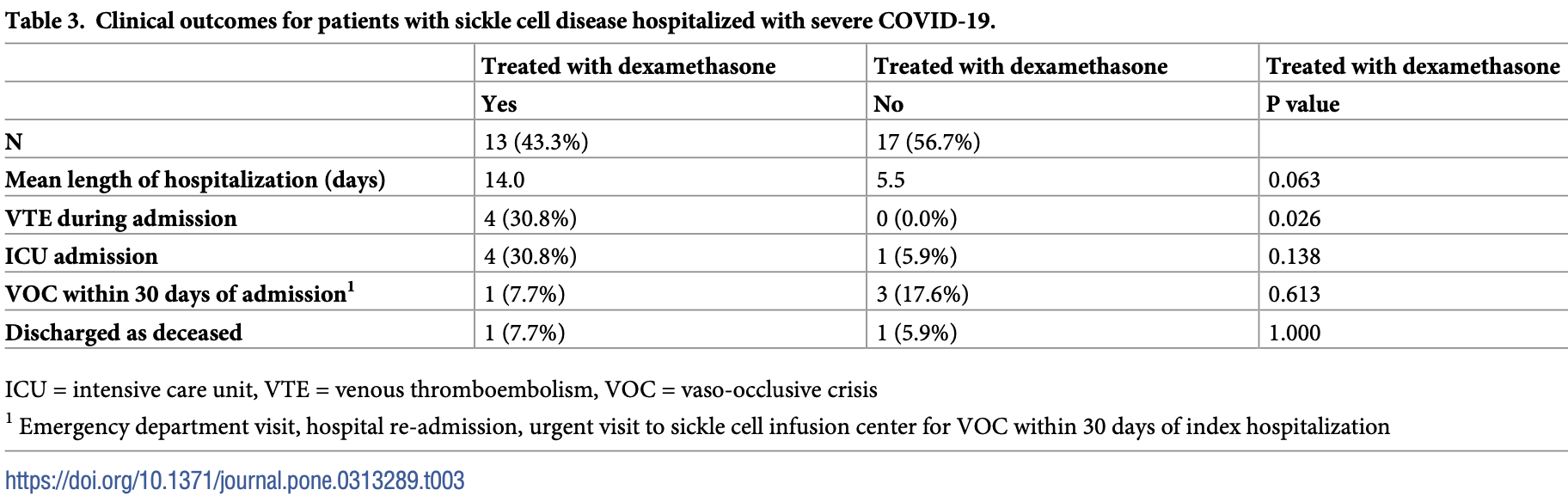
Retrospective 30 hospitalized patients with sickle cell disease (SCD) showing increased risk of venous thromboembolism (VTE) with dexamethasone treatment for COVID-19. There were also trends towards increased ICU admission and longer hospital stays with dexamethasone, without statistical significance.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 30.8% higher, RR 1.31, p = 1.00, treatment 1 of 13 (7.7%), control 1 of 17 (5.9%).
|
|
risk of ICU admission, 423.1% higher, RR 5.23, p = 0.14, treatment 4 of 13 (30.8%), control 1 of 17 (5.9%).
|
|
hospitalization time, 154.5% higher, relative time 2.55, p = 0.06, treatment 13, control 17.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Garneau et al., 26 Nov 2024, retrospective, USA, peer-reviewed, median age 33.7, 6 authors, study period 1 June, 2020 - 26 June, 2022.
Contact: william.garneau@jhmi.edu, jhmeirb@jhmi.edu.
Clinical effects of dexamethasone among patients with sickle cell disease hospitalized with COVID-19: Outcomes from a single academic health system
PLOS ONE, doi:10.1371/journal.pone.0313289
Background Dexamethasone is a steroid used in the treatment of hospitalized patients with severe COVID-19. However, the effect of dexamethasone in patients with SCD remains unclear given that steroids may precipitate vaso-occlusive crisis (VOC) in patients with SCD.
Methods and findings We performed a retrospective analysis of patients with SCD who were hospitalized at Johns Hopkins Health System between June 1, 2020 and June 26, 2022. We reviewed individual charts to assess severity of illness and eligibility for dexamethasone treatment. The exposure of interest was treatment with dexamethasone. Outcomes of interest included incident VTE, length of hospital stay, ICU admission, follow up-VOC and mortality. We identified 30 patients with SCD and COVID-19 who were eligible for dexamethasone treatment, 13 of whom received dexamethasone. Dexamethasone was associated with an increased risk of incident VTE (risk difference = 36%; 95% CI 8%, 66%) after adjustment for high-risk genotypes, >3 hospitalizations, and receipt of anticoagulation. There was an increase in the risk difference of ICU admission and an increased length of stay in crude and adjusted analyses however these associations were not statistically significant.
Conclusions We analyzed outcomes among patients with SCD who were hospitalized for COVID-19 and eligible for dexamethasone. Our study suggests that in this population, treatment with dexamethasone increases the risk of incident VTE. There was a suggestion of an increased risk
Author Contributions Conceptualization: William M. Garneau, Matthew J. Lankiewicz, Ashley P. Lauriello, Kelly A. Gebo, Sophie M. Lanzkron. Data curation: William M. Garneau, Matthew J. Lankiewicz, Kelly A. Gebo, Sophie M. Lanzkron. Formal analysis: William M. Garneau, Catherine R. Lesko, Kelly A. Gebo, Sophie M. Lanzkron. Investigation: William M. Garneau, Matthew J. Lankiewicz, Catherine R. Lesko, Ashley P. Lauriello, Kelly A. Gebo, Sophie M. Lanzkron. Methodology: William M. Garneau, Matthew J. Lankiewicz, Catherine R. Lesko, Kelly A. Gebo, Sophie M. Lanzkron. Project administration: William M. Garneau, Sophie M. Lanzkron. Resources: William M. Garneau, Kelly A. Gebo. Software: William M. Garneau, Catherine R. Lesko. Supervision: William M. Garneau, Kelly A. Gebo, Sophie M. Lanzkron. Validation: William M. Garneau, Matthew J. Lankiewicz, Catherine R. Lesko, Kelly A. Gebo. Visualization: William M. Garneau. Writing -original draft: William M. Garneau, Matthew J. Lankiewicz, Kelly A. Gebo, Sophie M. Lanzkron. Writing -review & editing: William M. Garneau, Matthew J. Lankiewicz, Catherine R. Lesko, Ashley P. Lauriello, Kelly A. Gebo, Sophie M. Lanzkron.
References
Alkindi, Elsadek, Al-Madhani, Al-Musalhi, Alkindi et al., Impact of COVID-19 on vasooclusive crisis in patients with sickle cell anaemia, Int J Infect Dis IJID Off Publ Int Soc Infect Dis, doi:10.1016/j.ijid.2021.03.044
Arlet, Lionnet, Khimoud, Joseph, Montalembert et al., Risk factors for severe COVID -19 in hospitalized sickle cell disease patients: A study of 319 patients in France, Am J Hematol, doi:10.1002/ajh.26432
Balanchivadze, Kudirka, Askar, Almadhoun, Kuriakose et al., Impact of COVID-19 Infection on 24 Patients with Sickle Cell Disease. One Center Urban Experience, Hemoglobin, doi:10.1080/03630269.2020.1797775
Calderwood, Sabir, Rao, Baker, Balasa et al., SARS-CoV-2 Infection Presenting as Acute Chest Syndrome in a Child With Hemoglobin SD-Los Angeles Disease: A Case Report and Review of Literature, J Pediatr Hematol Oncol, doi:10.1097/MPH.0000000000002546
Christian, Lanzkron, Naik, COVID-19 outcomes in sickle cell disease and sickle cell trait, Best Pract Res Clin Haematol, doi:10.1016/j.beha.2022.101382
Clift, Saatci, Coupland, Dambha-Miller, Cox, Sickle Cell Disorders and Severe COVID-19 Outcomes: A Cohort Study, Ann Intern Med, doi:10.7326/M21-1375
Cohen, Klings, Systemic Steroids and the Risk of Vasoocclusive Events in Patients with Sickle Cell Disease, Ann Am Thorac Soc
Efron, Tibshirani, An Introduction to the Bootstrap
Guarino, Caplan, Gbadebo, Jurkovitz, COVID-19 in Adults with Sickle Cell Disease, Blood
Herna ´n, ´ndez-Dı ´az, Werler, Mitchell, Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology, Am J Epidemiol, doi:10.1093/aje/155.2.176
Hoogenboom, Alamuri, Mcmahon, Balanchivadze, Dabak et al., Clinical outcomes of COVID-19 in patients with sickle cell disease and sickle cell trait: A critical appraisal of the literature, Blood Rev, doi:10.1016/j.blre.2021.100911
Hoogenboom, Fleysher, Soby, Mirhaji, Mitchell et al., Individuals with sickle cell disease and sickle cell trait demonstrate no increase in mortality or critical illness from COVID-19a fifteen hospital observational study in the Bronx, New York, Haematologica, doi:10.3324/haematol.2021.279222
Marshall, Murthy, Diaz, Adhikari, Angus et al., A minimal common outcome measure set for COVID-19 clinical research, Lancet Infect Dis, doi:10.1016/S1473-3099%2820%2930483-7
Martin, Darbari, Margulies, Nickel, Leonard et al., Clinical outcomes of children and adolescents with sickle cell disease and COVID-19 infection: A year in review at a metropolitan tertiary pediatric hospital, Front Med
Mucalo, Brandow, Dasgupta, Mason, Simpson et al., Comorbidities are risk factors for hospitalization and serious COVID-19 illness in children and adults with sickle cell disease, Blood Adv, doi:10.1182/bloodadvances.2021004288
Piel, Steinberg, Rees, Longo, Sickle Cell Disease, N Engl J Med
Walter, Cougoul, Maquet, Bartolucci, Lapeyre-Mestre et al., Risk of vaso-occlusive episode after exposure to corticosteroids in patients with sickle cell disease, Blood, doi:10.1182/blood.2021014473
DOI record:
{
"DOI": "10.1371/journal.pone.0313289",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0313289",
"abstract": "<jats:sec id=\"sec001\">\n<jats:title>Background</jats:title>\n<jats:p>Dexamethasone is a steroid used in the treatment of hospitalized patients with severe COVID-19. However, the effect of dexamethasone in patients with SCD remains unclear given that steroids may precipitate vaso-occlusive crisis (VOC) in patients with SCD.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec002\">\n<jats:title>Methods and findings</jats:title>\n<jats:p>We performed a retrospective analysis of patients with SCD who were hospitalized at Johns Hopkins Health System between June 1, 2020 and June 26, 2022. We reviewed individual charts to assess severity of illness and eligibility for dexamethasone treatment. The exposure of interest was treatment with dexamethasone. Outcomes of interest included incident VTE, length of hospital stay, ICU admission, follow up-VOC and mortality. We identified 30 patients with SCD and COVID-19 who were eligible for dexamethasone treatment, 13 of whom received dexamethasone. Dexamethasone was associated with an increased risk of incident VTE (risk difference = 36%; 95% CI 8%, 66%) after adjustment for high-risk genotypes, >3 hospitalizations, and receipt of anticoagulation. There was an increase in the risk difference of ICU admission and an increased length of stay in crude and adjusted analyses however these associations were not statistically significant.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec003\">\n<jats:title>Conclusions</jats:title>\n<jats:p>We analyzed outcomes among patients with SCD who were hospitalized for COVID-19 and eligible for dexamethasone. Our study suggests that in this population, treatment with dexamethasone increases the risk of incident VTE. There was a suggestion of an increased risk of ICU admission as well as increased length of hospitalization; larger studies are needed to confirm these findings.</jats:p>\n</jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1849-1640",
"affiliation": [],
"authenticated-orcid": true,
"family": "Garneau",
"given": "William M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lankiewicz",
"given": "Matthew J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lesko",
"given": "Catherine R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lauriello",
"given": "Ashley P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gebo",
"given": "Kelly A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lanzkron",
"given": "Sophie M.",
"sequence": "additional"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2024,
11,
26
]
],
"date-time": "2024-11-26T19:06:17Z",
"timestamp": 1732647977000
},
"deposited": {
"date-parts": [
[
2024,
11,
26
]
],
"date-time": "2024-11-26T19:06:26Z",
"timestamp": 1732647986000
},
"editor": [
{
"affiliation": [],
"family": "Saraf",
"given": "Santosh L.",
"sequence": "first"
}
],
"funder": [
{
"DOI": "10.13039/100006108",
"award": [
"KL2TR003099"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100006108",
"id-type": "DOI"
}
],
"name": "National Center for Advancing Translational Sciences"
},
{
"award": [
"1UL1TR001079-01"
],
"name": "Institute for Clinical and Translational Research"
}
],
"indexed": {
"date-parts": [
[
2024,
11,
27
]
],
"date-time": "2024-11-27T05:36:11Z",
"timestamp": 1732685771858,
"version": "3.28.2"
},
"is-referenced-by-count": 0,
"issue": "11",
"issued": {
"date-parts": [
[
2024,
11,
26
]
]
},
"journal-issue": {
"issue": "11",
"published-online": {
"date-parts": [
[
2024,
11,
26
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
11,
26
]
],
"date-time": "2024-11-26T00:00:00Z",
"timestamp": 1732579200000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0313289",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0313289",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2024,
11,
26
]
]
},
"published-online": {
"date-parts": [
[
2024,
11,
26
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"DOI": "10.1016/j.beha.2022.101382",
"article-title": "COVID-19 outcomes in sickle cell disease and sickle cell trait",
"author": "J Christian",
"doi-asserted-by": "crossref",
"first-page": "101382",
"issue": "3",
"journal-title": "Best Pract Res Clin Haematol",
"key": "pone.0313289.ref001",
"volume": "35",
"year": "2022"
},
{
"DOI": "10.1056/NEJMra1510865",
"article-title": "Sickle Cell Disease. Longo DL, editor",
"author": "FB Piel",
"doi-asserted-by": "crossref",
"first-page": "1561",
"issue": "16",
"journal-title": "N Engl J Med",
"key": "pone.0313289.ref002",
"volume": "376",
"year": "2017"
},
{
"article-title": "FDA Approves First Gene Therapies to Treat Patients with Sickle Cell Disease [",
"author": "Food and Drug Administration",
"key": "pone.0313289.ref003",
"year": "2023"
},
{
"article-title": "FDA approves first targeted therapy to treat patients with painful complication of sickle cell disease",
"author": "Food and Drug Administration",
"key": "pone.0313289.ref004",
"year": "2019"
},
{
"DOI": "10.1016/j.blre.2021.100911",
"article-title": "Clinical outcomes of COVID-19 in patients with sickle cell disease and sickle cell trait: A critical appraisal of the literature",
"author": "WS Hoogenboom",
"doi-asserted-by": "crossref",
"first-page": "100911",
"journal-title": "Blood Rev",
"key": "pone.0313289.ref005",
"volume": "53",
"year": "2022"
},
{
"DOI": "10.7326/M21-1375",
"article-title": "Sickle Cell Disorders and Severe COVID-19 Outcomes: A Cohort Study",
"author": "AK Clift",
"doi-asserted-by": "crossref",
"first-page": "1483",
"issue": "10",
"journal-title": "Ann Intern Med",
"key": "pone.0313289.ref006",
"volume": "174",
"year": "2021"
},
{
"article-title": "Risk factors for severe COVID ‐19 in hospitalized sickle cell disease patients: A study of 319 patients in France",
"author": "J Arlet",
"journal-title": "Am J Hematol",
"key": "pone.0313289.ref007",
"year": "2022"
},
{
"DOI": "10.1182/bloodadvances.2021004288",
"article-title": "Comorbidities are risk factors for hospitalization and serious COVID-19 illness in children and adults with sickle cell disease",
"author": "L Mucalo",
"doi-asserted-by": "crossref",
"first-page": "2717",
"issue": "13",
"journal-title": "Blood Adv",
"key": "pone.0313289.ref008",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.3324/haematol.2021.279222",
"article-title": "Individuals with sickle cell disease and sickle cell trait demonstrate no increase in mortality or critical illness from COVID-19—a fifteen hospital observational study in the Bronx, New York.",
"author": "WS Hoogenboom",
"doi-asserted-by": "crossref",
"first-page": "3014",
"issue": "11",
"journal-title": "Haematologica",
"key": "pone.0313289.ref009",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in Hospitalized Patients with Covid-19",
"author": "The RECOVERY Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "pone.0313289.ref010",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1182/blood.2021014473",
"article-title": "Risk of vaso-occlusive episode after exposure to corticosteroids in patients with sickle cell disease",
"author": "O Walter",
"doi-asserted-by": "crossref",
"first-page": "3771",
"issue": "26",
"journal-title": "Blood",
"key": "pone.0313289.ref011",
"volume": "139",
"year": "2022"
},
{
"author": "RT Cohen",
"first-page": "18",
"issue": "1",
"journal-title": "Systemic Steroids and the Risk of Vasoocclusive Events in Patients with Sickle Cell Disease. Ann Am Thorac Soc",
"key": "pone.0313289.ref012",
"volume": "20",
"year": "2023"
},
{
"article-title": "COVID-19 PRECISION MEDICINE ANALYTICS PLATFORM REGISTRY (JH-CROWN) [Internet]",
"author": "Johns Hopkins Institute for Clinical and Translational Research",
"key": "pone.0313289.ref013"
},
{
"article-title": "Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19",
"author": "The RECOVERY Collaborative Group",
"key": "pone.0313289.ref014",
"year": "2020"
},
{
"article-title": "COVID-19 OUTPATIENT TREATMENT GUIDELINES ROADMAP",
"author": "Infectious Diseases Society of America",
"key": "pone.0313289.ref015"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"article-title": "A minimal common outcome measure set for COVID-19 clinical research",
"author": "JC Marshall",
"doi-asserted-by": "crossref",
"first-page": "e192",
"issue": "8",
"journal-title": "Lancet Infect Dis",
"key": "pone.0313289.ref016",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1093/aje/155.2.176",
"article-title": "Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology",
"author": "MA Hernán",
"doi-asserted-by": "crossref",
"first-page": "176",
"issue": "2",
"journal-title": "Am J Epidemiol",
"key": "pone.0313289.ref017",
"volume": "155",
"year": "2002"
},
{
"author": "B Efron",
"key": "pone.0313289.ref018",
"volume-title": "Monographs on Statistics and Applied Probability",
"year": "1993"
},
{
"DOI": "10.1182/blood-2023-177681",
"article-title": "COVID-19 in Adults with Sickle Cell Disease",
"author": "SH Guarino",
"doi-asserted-by": "crossref",
"first-page": "2373",
"issue": "Supplement 1",
"journal-title": "Blood",
"key": "pone.0313289.ref019",
"volume": "142",
"year": "2023"
},
{
"DOI": "10.1097/MPH.0000000000002546",
"article-title": "SARS-CoV-2 Infection Presenting as Acute Chest Syndrome in a Child With Hemoglobin SD-Los Angeles Disease: A Case Report and Review of Literature",
"author": "S Calderwood",
"doi-asserted-by": "crossref",
"first-page": "82",
"issue": "2",
"journal-title": "J Pediatr Hematol Oncol",
"key": "pone.0313289.ref020",
"volume": "45",
"year": "2023"
},
{
"DOI": "10.3389/fmed.2023.987194",
"article-title": "Clinical outcomes of children and adolescents with sickle cell disease and COVID-19 infection: A year in review at a metropolitan tertiary pediatric hospital.",
"author": "OY Martin",
"doi-asserted-by": "crossref",
"first-page": "987194",
"journal-title": "Front Med.",
"key": "pone.0313289.ref021",
"volume": "10",
"year": "2023"
},
{
"article-title": "Impact of COVID-19 on vasooclusive crisis in patients with sickle cell anaemia",
"author": "S Alkindi",
"first-page": "128",
"journal-title": "Int J Infect Dis IJID Off Publ Int Soc Infect Dis",
"key": "pone.0313289.ref022",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.1080/03630269.2020.1797775",
"article-title": "Impact of COVID-19 Infection on 24 Patients with Sickle Cell Disease. One Center Urban Experience, Detroit, MI, USA.",
"author": "N Balanchivadze",
"doi-asserted-by": "crossref",
"first-page": "284",
"issue": "4",
"journal-title": "Hemoglobin",
"key": "pone.0313289.ref023",
"volume": "44",
"year": "2020"
},
{
"article-title": "COVID-19 Resources. [cited 2024 Apr 7]. COVID-19 and Sickle Cell Disease: Frequently Asked Questions",
"author": "American Society of Hematology",
"key": "pone.0313289.ref024"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0313289"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Clinical effects of dexamethasone among patients with sickle cell disease hospitalized with COVID-19: Outcomes from a single academic health system",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "19"
}