A Pilot of a Randomized Control Trial of Melatonin and Vitamin C for Mild-to-Moderate COVID-19
et al., The Journal of the American Board of Family Medicine, doi:10.3122/jabfm.2022.04.210529, NCT04530539, Jul 2022
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000076 from 73 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
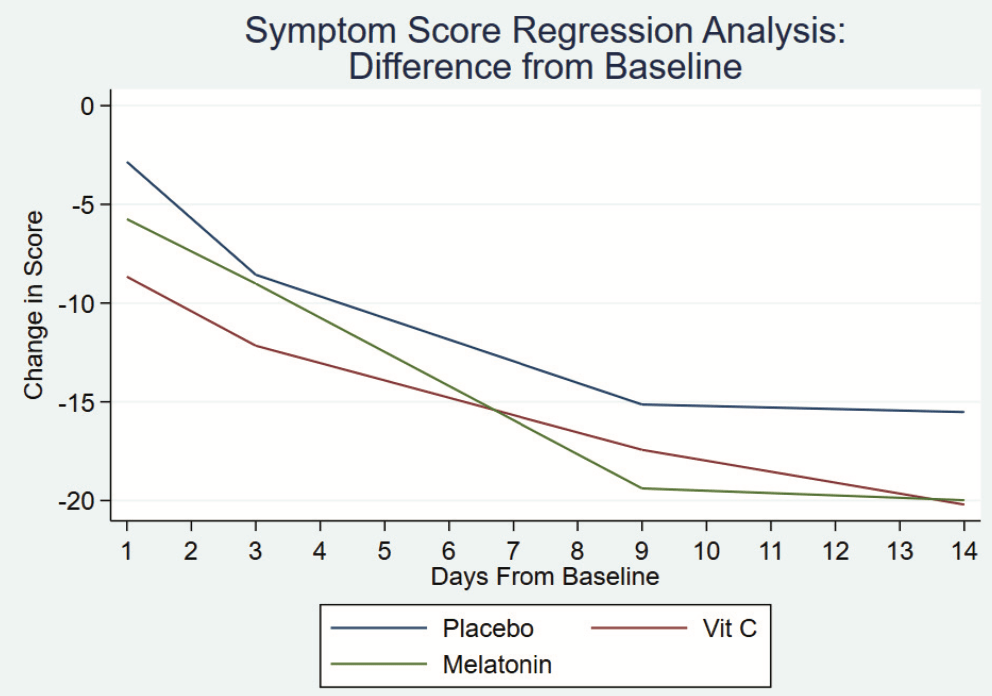
Early terminated low-risk patient RCT with 32 low-dose vitamin C, 32 melatonin, and 34 placebo patients, showing faster resolution of symptoms with melatonin in spline regression analysis, and no significant difference for vitamin C. All patients recovered with no serious outcomes reported. Baseline symptoms scores were higher in the melatonin and vitamin C arms (median 27 and 24 vs. 18 for placebo).
This is the 12th of 20 COVID-19 RCTs for vitamin C, which collectively show efficacy with p=0.0016.
This is the 48th of 73 COVID-19 controlled studies for vitamin C, which collectively show efficacy with p=0.000000076.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers vitamin C and melatonin.
|
relative recovery, 4.4% better, RR 0.96, p = 0.83, treatment mean 17.59 (±13.1) n=32, control mean 16.82 (±15.7) n=34, mid-recovery, relative symptom improvement, day 9.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Fogleman et al., 27 Jul 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, mean age 52.0, 7 authors, study period 5 October, 2020 - 21 June, 2021, average treatment delay 6.0 days, dosage 1000mg days 1-14, trial NCT04530539 (history).
Contact: corey.fogleman@pennmedicine.upenn.edu.
A Pilot of a Randomized Control Trial of Melatonin and Vitamin C for Mild-to-Moderate COVID-19
The Journal of the American Board of Family Medicine, doi:10.3122/jabfm.2022.04.210529
This study aimed to help determine the effect of dietary supplements on symptom course and quality of life in patients with mild-to-moderate COVID-19 infection. Design: We modified the Wisconsin Upper Respiratory Symptom Survey (WURSS) to conduct a 3 arm, parallel, randomized, double-blind, placebo-controlled trial, enrolling patients with mild-to-moderate symptoms of COVID-19 infection. Patients took placebo (n = 34), vitamin C 1000 mg (n = 32), or melatonin 10 mg (n = 32) orally for 14 days. Outcomes: Ninety Eight (98 out of 104 recruited; mean age = 52 years) patients completed the study. Outcomes were calculated as differences from baseline scores on each of 2 WURSS-derived surveys and analyzed using a spline regression analysis. Regarding symptom progression, those patients taking placebo and vitamin C progressed at the same rate. When compared with those taking placebo (coefficient = -1.09 (95% confidence interval [CI] = -1.39 to -0.8) the group taking melatonin had a faster resolution of symptoms (coefficient = -0.63 [95% CI -1.02 to -0.21] P = .003). By day 14 all 3 groups had reached plateau. Quality-of-life impact analysis demonstrated that the group taking vitamin C improved at the same rate as the group taking placebo (coefficient = -0.71 (95% CI = -1.11 to -0.3)). The group taking melatonin (coefficient = -1.16 (95% CI = -1.75 to -0.57) P < .005) had a faster improvement in quality-oflife. By day 14 all 3 groups had reached plateau. Conclusion: Vitamin C 1000 mg once daily has no effect on disease progression. Melatonin 10 mg daily may have a statistically significant effect but it is unclear if this represents a clinically significant benefit to those with mild-to-moderate symptoms of COVID-19 infection. Further study is warranted. ( J Am Board Fam Med 2022;35:695-707.
References
Alamdari, Moghaddam, Amini, Application of methylene blue-vitamin C-N-acetyl cysteine for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial, Eur J Pharmacol
Barrett, Brown, Mundt, The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid, J Clin Epidemiol
Barrett, Brown, Mundt, Validation of a short form Wisconsin Upper Respiratory Symptom Survey (WURSS-21), Health Qual Life Outcomes
Brown, Pandi-Perumal, Pupko, Kennedy, Cardinali, Melatonin as an Add-On Treatment of COVID-19 Infection: Current Status, Diseases
Brown, Pandi-Perumal, Pupko, Kennedy, Cardinali, Melatonin as an Add-On Treatment of COVID-19 Infection: Current Status, Diseases
Cai, Li, Tang, A New Mechanism of Vitamin C Effects on A/FM/1/47(H1N1) Virus-Induced Pneumonia in Restraint-Stressed Mice, Biomed Res Int
Cross, Landis, Sehgal, Payne, Melatonin for the Early Treatment of COVID-19: A Narrative Review of Current Evidence and Possible Efficacy, Endocr Pract
Farnoosh, Akbariqomi, Badri, Efficacy of a Low Dose of Melatonin as an Adjunctive Therapy in Hospitalized Patients with COVID-19: A Randomized, Double-blind Clin-ical Trial, Arch Med Res
Hardeland, Melatonin and inflammation -Story of a Double-Edged Blade, J Pineal Res
Hemilä, Chalker, Cochrane, Respiratory, Infections Group. Vitamin C for preventing and treating the common cold, Cochrane Database Syst Rev, doi:10.1002/14651858.CD000980.pub4/full
Hemilä, Chalker, Douglas, Vitamin C for preventing and treating the common cold, Cochrane Database Syst Rev, doi:10.1002/14651858.CD000980.pub3/information
Hiedra, Lo, Elbashabsheh, The use of IV vitamin C for patients with COVID-19: A case series, Expert Rev. Anti-Infect. Ther
Jamalimoghadamsiahkali, Zarezade, Koolaji, Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized openlabel clinical trial, Eur J Med Res
Jovic, Ali, Ibrahim, Could Vitamins Help in the Fight Against COVID-19?, Nutrients
Kashiouris, Heureux, Cable, Fisher, Leichtle et al., The Emerging Role of Vitamin C as a Treatment for Sepsis, Nutrients
Mousavi, Heydari, Mehravaran, Melatonin effects on sleep quality and outcomes of COVID-19 patients: An open-label, randomized, controlled trial, J Med Virol
Ramlall, Zucker, Tatonetti, Melatonin is significantly associated with survival of intubated COVID-19 patients, medRxiv
Rawat, Roy, Maitra, Gulati, Khanna et al., Vitamin C and COVID-19 treatment: A systematic review and meta-analysis of randomized controlled trials, Diabetes Metab Syndr
Silvestri, Rossi, Melatonin: its possible role in the management of viral infections -a brief review, Ital J Pediatr
Thomas, Patel, Bittel, Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial, JAMA Netw Open
Ye, Wang, Mao, The Pathogenesis and Treatment of the 'Cytokine Storm' in COVID-19, J Infect
Zhang, Wang, Ni, COVID-19: Melatonin as a potential adjuvant treatment, Life Sci
Zhou, Hou, Shen, A network medicine approach to investigation and population-based validation of disease manifestations and drug repurposing for COVID-19, PLoS Biol
Öztürk, Akbulut, Güney, Melatonin, aging, and COVID-19: Could melatonin be beneficial for COVID-19 treatment in the elderly?, Turk J Med Sci
DOI record:
{
"DOI": "10.3122/jabfm.2022.04.210529",
"ISSN": [
"1557-2625",
"1558-7118"
],
"URL": "http://dx.doi.org/10.3122/jabfm.2022.04.210529",
"alternative-id": [
"10.3122/jabfm.2022.04.210529"
],
"author": [
{
"affiliation": [],
"family": "Fogleman",
"given": "Corey",
"sequence": "first"
},
{
"affiliation": [],
"family": "Cohen",
"given": "Donna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mercier",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farrell",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rutz",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bresz",
"given": "Kellie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vernon",
"given": "Tawnya",
"sequence": "additional"
}
],
"container-title": "The Journal of the American Board of Family\n Medicine",
"container-title-short": "J Am Board Fam\n Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
7,
28
]
],
"date-time": "2022-07-28T06:45:30Z",
"timestamp": 1658990730000
},
"deposited": {
"date-parts": [
[
2022,
7,
28
]
],
"date-time": "2022-07-28T06:45:47Z",
"timestamp": 1658990747000
},
"indexed": {
"date-parts": [
[
2022,
7,
29
]
],
"date-time": "2022-07-29T04:47:17Z",
"timestamp": 1659070037565
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2022,
7
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2022,
7,
27
]
]
},
"published-print": {
"date-parts": [
[
2022,
7
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.3122/jabfm.2022.04.210529",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1658",
"original-title": [],
"page": "695-707",
"prefix": "10.3122",
"published": {
"date-parts": [
[
2022,
7
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
27
]
]
},
"published-print": {
"date-parts": [
[
2022,
7
]
]
},
"publisher": "American Board of Family Medicine (ABFM)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.jabfm.org/lookup/doi/10.3122/jabfm.2022.04.210529"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Family Practice",
"Public Health, Environmental and Occupational Health"
],
"subtitle": [],
"title": "A Pilot of a Randomized Control Trial of Melatonin and Vitamin C for Mild-to-Moderate COVID-19",
"type": "journal-article",
"volume": "35"
}
fogleman
