Report of the first seven agents in the I-SPY COVID trial: a phase 2, open label, adaptive platform randomised controlled trial
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2023.101889, I-SPY COVID, NCT04488081, Mar 2023
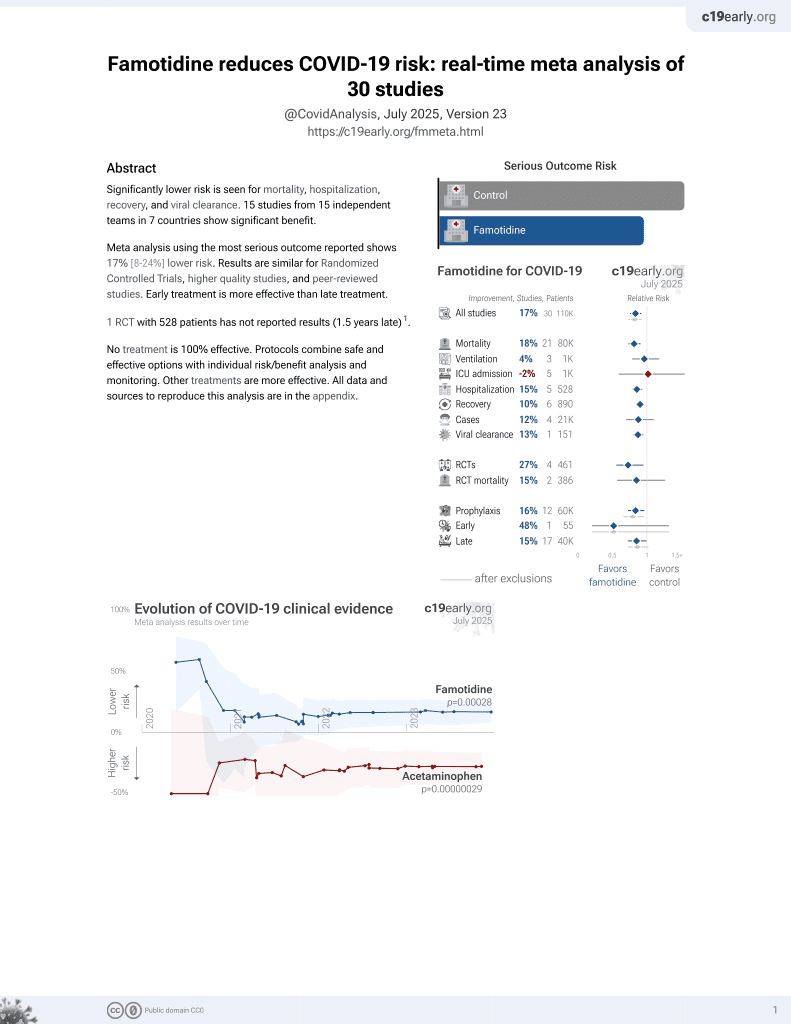
Famotidine for COVID-19
28th treatment shown to reduce risk in
October 2021, now with p = 0.00028 from 30 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
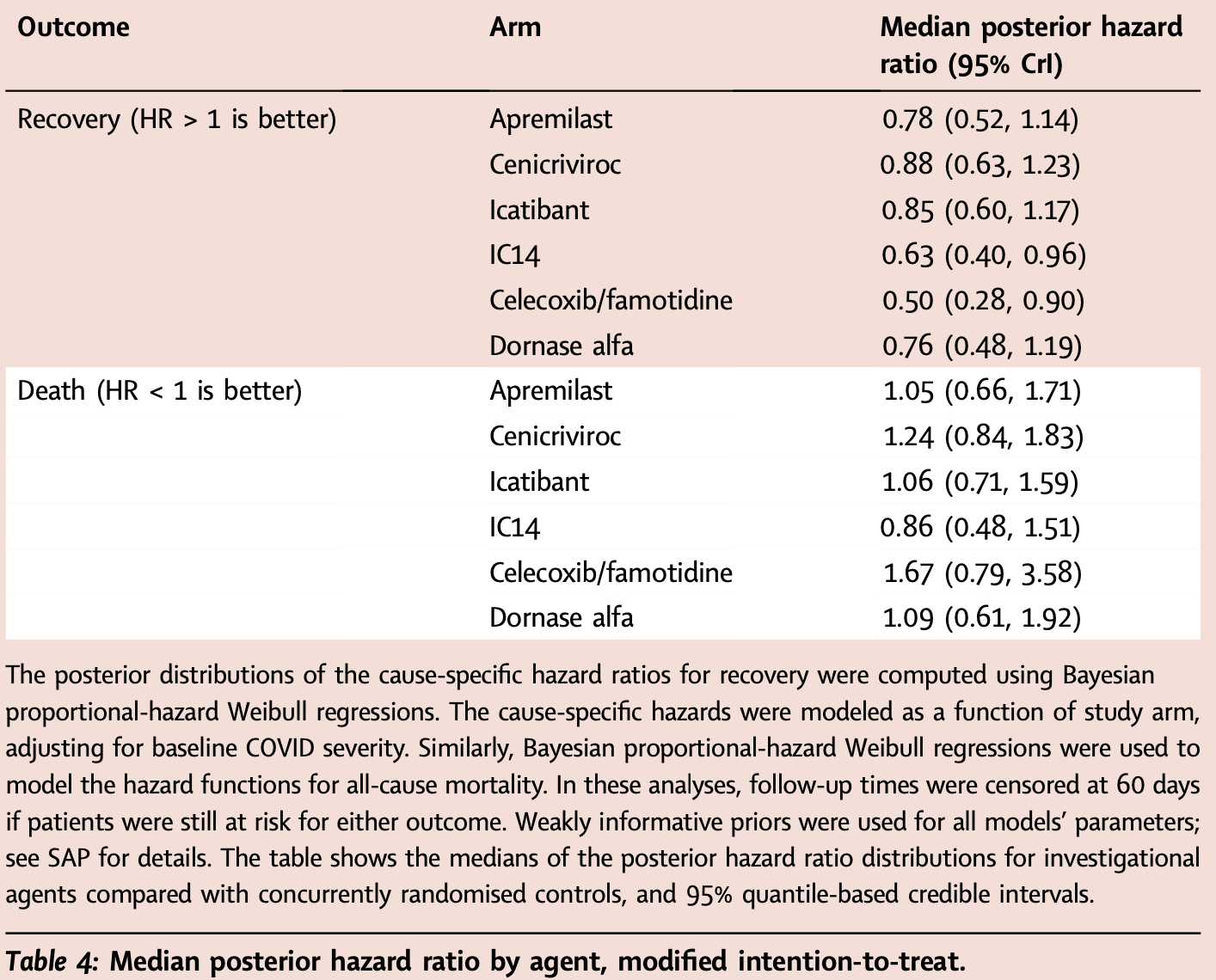
RCT severe COVID-19 patients requiring ≥6 L/min oxygen, showing worse recovery with the addition of celecoxib and famotidine to remdesivir and dexamethasone. The treatment group mean age was 9 years older, and the treatment group had more patients on high-flow nasal oxygen or ventilation at baseline (27% to 14%). Remdesivir and celecoxib have shown nephrotoxicity, hepatotoxicity, and cardiotoxicity, and the combination of both in late stage patients may further increase risk. Authors defined AKI as an adverse events of special interest for celecoxib, but do not report results (only RRT which depends on clinical condition and survival, and is only reported including non-concurrent controls). Celecoxib 400mg BID for 7 days, famotidine 80mg QID for 7 days followed by 40mg BID for 14 days. Publication was delayed over 1.5 years after completion without explanation.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
This study is excluded in meta-analysis:
contribution of famotidine unclear with combined treatment, remdesivir and celecoxib have shown nephrotoxicity, hepatotoxicity, and cardiotoxicity, and the combination of both in late stage patients may further increase risk.
|
risk of death, 67.0% higher, HR 1.67, p = 0.18, treatment 12 of 30 (40.0%), control 8 of 37 (21.6%), adjusted per study, day 60.
|
|
risk of no recovery, 100% higher, HR 2.00, p = 0.02, treatment 15 of 30 (50.0%), control 8 of 37 (21.6%), adjusted per study, inverted to make HR<1 favor treatment, day 60.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Files et al., 3 Mar 2023, Randomized Controlled Trial, USA, peer-reviewed, 91 authors, study period 30 July, 2020 - 11 June, 2021, average treatment delay 9.5 days, this trial uses multiple treatments in the treatment arm (combined with celecoxib) - results of individual treatments may vary, trial NCT04488081 (history) (I-SPY COVID).
Report of the first seven agents in the I-SPY COVID trial: a phase 2, open label, adaptive platform randomised controlled trial
eClinicalMedicine, doi:10.1016/j.eclinm.2023.101889
Background An urgent need exists to rapidly screen potential therapeutics for severe COVID-19 or other emerging pathogens associated with high morbidity and mortality. Methods Using an adaptive platform design created to rapidly evaluate investigational agents, hospitalised patients with severe COVID-19 requiring ≥6 L/min oxygen were randomised to either a backbone regimen of dexamethasone and remdesivir alone (controls) or backbone plus one open-label investigational agent. Patients were enrolled to the arms described between July 30, 2020 and June 11, 2021 in 20 medical centres in the United States. The platform contained up to four potentially available investigational agents and controls available for randomisation during a single time-period. The two primary endpoints were time-to-recovery (<6 L/min oxygen for two consecutive days) and mortality. Data were evaluated biweekly in comparison to pre-specified criteria for graduation (i.e., likely efficacy), futility, and safety, with an adaptive sample size of 40-125 individuals per agent and a Bayesian analytical approach. Criteria were designed to achieve rapid screening of agents and to identify large benefit signals. Concurrently enrolled controls were used for all analyses. https://clinicaltrials.gov/ct2/show/NCT04488081. Findings The first 7 agents evaluated were cenicriviroc (CCR2/5 antagonist; n = 92), icatibant (bradykinin antagonist; n = 96), apremilast (PDE4 inhibitor; n = 67), celecoxib/famotidine (COX2/histamine blockade; n = 30), IC14 (anti-CD14; n = 67), dornase alfa (inhaled DNase; n = 39) and razuprotafib (Tie2 agonist; n = 22). Razuprotafib was dropped from the trial due to feasibility issues. In the modified intention-to-treat analyses, no agent met pre-specified efficacy/ graduation endpoints with posterior probabilities for the hazard ratios [HRs] for recovery ≤1.5 between 0.99 and 1.00. The data monitoring committee stopped Celecoxib/Famotidine for potential harm (median posterior HR for recovery 0.5, 95% credible interval [CrI] 0.28-0.90; median posterior HR for death 1.67, 95% CrI 0.79-3.58). Interpretation None of the first 7 agents to enter the trial met the prespecified criteria for a large efficacy signal. Celecoxib/Famotidine was stopped early for potential harm. Adaptive platform trials may provide a useful approach to rapidly screen multiple agents during a pandemic.
Appendix A. Supplementary data Supplementary data related to this article can be found at https://doi. org/10.1016/j.eclinm.2023.101889.
References
Angus, Derde, Al-Beidh, Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial, JAMA
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 -final report, N Engl J Med
Calfee, Clinical trial design during and beyond the pandemic: the I-SPY COVID trial, Nat Med
Douin, Siegel, Grandits, Evaluating primary endpoints for COVID-19 therapeutic trials to assess recovery, Am J Respir Crit Care Med
Files, Matthay, Calfee, I-SPY COVID adaptive platform trial for COVID-19 acute respiratory failure: rationale, design and operations, BMJ Open
Griffiths, Fitzgerald, Jaki, AGILE-ACCORD: a randomized, multicentre, seamless, adaptive phase I/II platform study to determine the optimal dose, safety and efficacy of multiple candidate agents for the treatment of COVID-19: a structured summary of a study protocol for a randomised platform trial, Trials
Group, Horby, Lim, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med
Matthay, Thompson, Ware, The Berlin definition of acute respiratory distress syndrome: should patients receiving highflow nasal oxygen be included?, Lancet Respir Med
Organization, WHO R&D blueprint: COVID-19 therapeutic trial synopsis
Sinha, Furfaro, Cummings, Latent class analysis reveals COVID-19-related acute respiratory distress syndrome subgroups with differential responses to corticosteroids, Am J Respir Crit Care Med
Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet
Woodcock, Araojo, Thompson, Puckrein, Integrating research into community practice -toward increased diversity in clinical trials, N Engl J Med
Woodcock, Lavange, Master protocols to study multiple therapies, multiple diseases, or both, N Engl J Med
DOI record:
{
"DOI": "10.1016/j.eclinm.2023.101889",
"ISSN": [
"2589-5370"
],
"URL": "http://dx.doi.org/10.1016/j.eclinm.2023.101889",
"alternative-id": [
"S2589537023000664"
],
"article-number": "101889",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Report of the first seven agents in the I-SPY COVID trial: a phase 2, open label, adaptive platform randomised controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "eClinicalMedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.eclinm.2023.101889"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 The Author. Published by Elsevier Ltd."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1595-4080",
"affiliation": [],
"authenticated-orcid": false,
"family": "Files",
"given": "D. Clark",
"sequence": "first"
},
{
"affiliation": [],
"family": "Aggarwal",
"given": "Neil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Albertson",
"given": "Timothy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Auld",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beitler",
"given": "Jeremy R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berger",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burnham",
"given": "Ellen L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Calfee",
"given": "Carolyn S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cobb",
"given": "Nathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crippa",
"given": "Alessio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Discacciati",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eklund",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Esserman",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Friedman",
"given": "Eliot",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gandotra",
"given": "Sheetal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "Kashif",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koff",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Santhi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Kathleen D.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8193-909X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Martin",
"given": "Thomas R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matthay",
"given": "Michael A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meyer",
"given": "Nuala J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Obermiller",
"given": "Timothy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robinson",
"given": "Philip",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Russell",
"given": "Derek",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thomas",
"given": "Karl",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wong",
"given": "Se Fum",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wunderink",
"given": "Richard G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wurfel",
"given": "Mark M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yen",
"given": "Albert",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6123-970X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Youssef",
"given": "Fady A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Darmanian",
"given": "Anita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dzierba",
"given": "Amy L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcia",
"given": "Ivan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gosek",
"given": "Katarzyna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Madahar",
"given": "Purnema",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mittel",
"given": "Aaron M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muir",
"given": "Justin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rosen",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schicchi",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Serra",
"given": "Alexis L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wahab",
"given": "Romina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gibbs",
"given": "Kevin W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Landreth",
"given": "Leigha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "LaRose",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parks",
"given": "Lisa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7256-5815",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wynn",
"given": "Adina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ittner",
"given": "Caroline A.G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mangalmurti",
"given": "Nilman S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3937-5320",
"affiliation": [],
"authenticated-orcid": false,
"family": "Reilly",
"given": "John P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harris",
"given": "Donna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Methukupally",
"given": "Abhishek",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Siddharth",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7150-7157",
"affiliation": [],
"authenticated-orcid": false,
"family": "Boerger",
"given": "Lindsie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kazianis",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Higgins",
"given": "Carrie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McKeehan",
"given": "Jeff",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daniel",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fields",
"given": "Scott",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hurst-Hopf",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jauregui",
"given": "Alejandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brown Swigart",
"given": "Lamorna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Blevins",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nguyen",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suarez",
"given": "Alexis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tanios",
"given": "Maged A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sarafian",
"given": "Farjad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shah",
"given": "Usman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adelman",
"given": "Max",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Creel-Bulos",
"given": "Christina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Detelich",
"given": "Joshua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harris",
"given": "Gavin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nugent",
"given": "Katherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Spainhour",
"given": "Christina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5142-1137",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yang",
"given": "Philip",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1301-4941",
"affiliation": [],
"authenticated-orcid": false,
"family": "Haczku",
"given": "Angela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hardy",
"given": "Erin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harper",
"given": "Richart",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morrissey",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sandrock",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Budinger",
"given": "G. R. Scott",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8827-8581",
"affiliation": [],
"authenticated-orcid": false,
"family": "Donnelly",
"given": "Helen K.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5775-8427",
"affiliation": [],
"authenticated-orcid": false,
"family": "Singer",
"given": "Benjamin D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moskowitz",
"given": "Ari",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coleman",
"given": "Melissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Levitt",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Ruixiao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Henderson",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asare",
"given": "Adam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dunn",
"given": "Imogene",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Botello Barragan",
"given": "Alejandro",
"sequence": "additional"
}
],
"container-title": "eClinicalMedicine",
"container-title-short": "eClinicalMedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
3,
3
]
],
"date-time": "2023-03-03T03:52:02Z",
"timestamp": 1677815522000
},
"deposited": {
"date-parts": [
[
2023,
5,
13
]
],
"date-time": "2023-05-13T17:07:47Z",
"timestamp": 1683997667000
},
"indexed": {
"date-parts": [
[
2023,
5,
13
]
],
"date-time": "2023-05-13T17:41:52Z",
"timestamp": 1683999712082
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
4
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
4,
1
]
],
"date-time": "2023-04-01T00:00:00Z",
"timestamp": 1680307200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
2,
14
]
],
"date-time": "2023-02-14T00:00:00Z",
"timestamp": 1676332800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537023000664?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537023000664?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "101889",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
4
]
]
},
"published-print": {
"date-parts": [
[
2023,
4
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19",
"author": "Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2023.101889_bib1",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17022",
"article-title": "Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial",
"author": "Angus",
"doi-asserted-by": "crossref",
"first-page": "1317",
"issue": "13",
"journal-title": "JAMA",
"key": "10.1016/j.eclinm.2023.101889_bib2",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMp2107331",
"article-title": "Integrating research into community practice - toward increased diversity in clinical trials",
"author": "Woodcock",
"doi-asserted-by": "crossref",
"first-page": "1351",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2023.101889_bib3",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1186/s13063-020-04473-1",
"author": "Griffiths",
"doi-asserted-by": "crossref",
"first-page": "544",
"issue": "1",
"journal-title": "Trials",
"key": "10.1016/j.eclinm.2023.101889_bib4",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1038/s41591-021-01617-x",
"article-title": "Clinical trial design during and beyond the pandemic: the I-SPY COVID trial",
"author": "Calfee",
"doi-asserted-by": "crossref",
"first-page": "9",
"issue": "1",
"journal-title": "Nat Med",
"key": "10.1016/j.eclinm.2023.101889_bib5",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1136/bmjopen-2021-060664",
"article-title": "I-SPY COVID adaptive platform trial for COVID-19 acute respiratory failure: rationale, design and operations",
"author": "Files",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "BMJ Open",
"key": "10.1016/j.eclinm.2023.101889_bib6",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19 - final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2023.101889_bib7",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1569",
"issue": "10236",
"journal-title": "Lancet",
"key": "10.1016/j.eclinm.2023.101889_bib8",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056/NEJMra1510062",
"article-title": "Master protocols to study multiple therapies, multiple diseases, or both",
"author": "Woodcock",
"doi-asserted-by": "crossref",
"first-page": "62",
"issue": "1",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2023.101889_bib9",
"volume": "377",
"year": "2017"
},
{
"author": "Organization",
"key": "10.1016/j.eclinm.2023.101889_bib10",
"series-title": "WHO R&D blueprint: COVID-19 therapeutic trial synopsis",
"year": "2020"
},
{
"DOI": "10.1164/rccm.202112-2836OC",
"article-title": "Evaluating primary endpoints for COVID-19 therapeutic trials to assess recovery",
"author": "Douin",
"doi-asserted-by": "crossref",
"first-page": "730",
"issue": "6",
"journal-title": "Am J Respir Crit Care Med",
"key": "10.1016/j.eclinm.2023.101889_bib11",
"volume": "206",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(22)00215-6",
"article-title": "Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial",
"doi-asserted-by": "crossref",
"first-page": "972",
"issue": "10",
"journal-title": "Lancet Respir Med",
"key": "10.1016/j.eclinm.2023.101889_bib12",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(21)00105-3",
"article-title": "The Berlin definition of acute respiratory distress syndrome: should patients receiving high-flow nasal oxygen be included?",
"author": "Matthay",
"doi-asserted-by": "crossref",
"first-page": "933",
"issue": "8",
"journal-title": "Lancet Respir Med",
"key": "10.1016/j.eclinm.2023.101889_bib13",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1164/rccm.202105-1302OC",
"article-title": "Latent class analysis reveals COVID-19-related acute respiratory distress syndrome subgroups with differential responses to corticosteroids",
"author": "Sinha",
"doi-asserted-by": "crossref",
"first-page": "1274",
"issue": "11",
"journal-title": "Am J Respir Crit Care Med",
"key": "10.1016/j.eclinm.2023.101889_bib14",
"volume": "204",
"year": "2021"
}
],
"reference-count": 14,
"references-count": 14,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2589537023000664"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Report of the first seven agents in the I-SPY COVID trial: a phase 2, open label, adaptive platform randomised controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "58"
}