Intranasal Chlorpheniramine for Early Symptomatic Treatment of COVID-19 and the Impact on Long-COVID
et al., Cureus, doi:10.7759/cureus.82736, Apr 2025
47th treatment shown to reduce risk in
December 2022, now with p < 0.00000000001 from 3 studies.
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
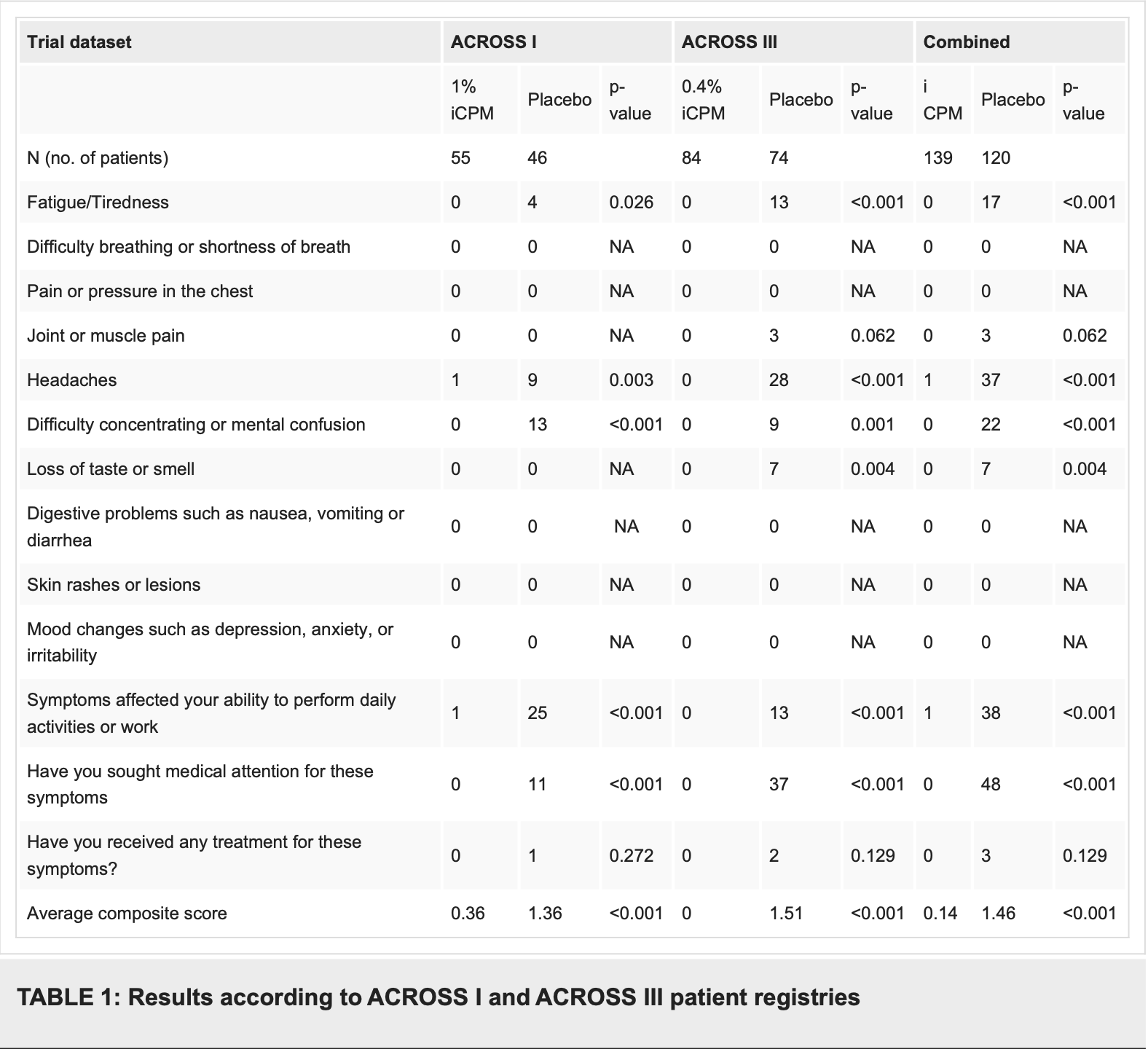
Review of intranasal chlorpheniramine maleate (iCPM) as a therapy for COVID-19 and long COVID. Authors explore how iCPM combines antihistamine activity via H1 receptor antagonism with bitter taste receptor (T2R) activation to address both acute infection and persistent symptoms. By inhibiting histamine-mediated inflammation and enhancing mucosal immunity, iCPM demonstrates significant potential for reducing viral replication, alleviating respiratory symptoms, and preventing progression to severe disease. Clinical evidence from the ACCROS trials shows that iCPM significantly reduced symptom severity and duration in acute COVID-19 patients compared to placebo, with particular improvements in sensory deficits and upper respiratory symptoms. Additionally, ACCROS III data revealed that none of the participants receiving iCPM reported persistent fatigue or mental confusion, compared to 14.2% and 18.3% in the placebo group. The intranasal delivery method allows for targeted action at the primary site of viral entry while minimizing systemic side effects. Authors propose that iCPM's mechanism of action includes inhibition of viral adsorption, suppression of viral replication, direct virucidal effects, and modulation of inflammatory pathways through NF-κB signaling.
See Ricke et al. for another review covering chlorpheniramine for COVID-19.
Ferrer et al., 21 Apr 2025, USA, peer-reviewed, 5 authors.
Contact: cesar_alas10@hotmail.com.
Intranasal Chlorpheniramine for Early Symptomatic Treatment of COVID-19 and the Impact on Long-COVID
Cureus, doi:10.7759/cureus.82736
This review explores the therapeutic potential of intranasal chlorpheniramine maleate (iCPM) in managing both acute COVID-19 and Long COVID by integrating histamine H1 receptor antagonism and bitter taste receptor (T2R) activation. Current literature on histamine-mediated inflammation, T2R activation, and the dual-action mechanisms of iCPM were analyzed. Emphasis was placed on its antiviral, anti-inflammatory, and mucosal immunity-enhancing properties. iCPM demonstrates significant efficacy in addressing acute COVID-19 symptoms by inhibiting histamine-mediated inflammatory pathways and reducing cytokine storms. As a T2R agonist, it enhances mucosal immunity through nitric oxide production, mucociliary clearance, and antimicrobial peptide synthesis, reducing viral replication and supporting respiratory health. Additionally, iCPM shows promise in mitigating persistent symptoms of long COVID, including fatigue, brain fog, and respiratory dysfunction, by addressing chronic inflammation and residual viral activity. The integration of H1 receptor antagonism and T2R activation positions iCPM as a novel dual-target therapy for respiratory infections. Its localized delivery and broad mechanism of action make it a promising candidate for managing both the acute and chronic phases of COVID-19. Future research should focus on large-scale clinical trials and personalized approaches based on genetic variations in T2R pathways.
Additional Information Author Contributions All authors have reviewed the final version to be published and agreed to be accountable for all aspects of the work.
Acquisition, analysis, or interpretation of data:
Disclosures Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: Kristhel Gaitán-Zambrano, Dennis J. Pavón-Varela declare(s) employment from Dr. Ferrer Biopharma. Kristhel Gaitán-Zambrano, and Dennis J. Pavón-Varela are affiliated with Dr. Ferrer Biopharma. Their involvement in this research is within their professional capacities at the institution. The study was conducted with scientific integrity, and the conclusions presented are based on an objective analysis of the data. No additional financial incentives, grants, or external funding directly related to this research were received outside of standard employment agreements. Any potential influence on the interpretation of results has been mitigated through adherence to ethical research guidelines and transparency in reporting. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References
Barham, Taha, Hall, Does phenotypic expression of bitter taste receptor T2R38 show association with COVID-19 severity?, Int Forum Allergy Rhinol, doi:10.1002/alr.22692
Bethineedi, Baghsheikhi, Soltani, Human T2R38 bitter taste receptor expression and COVID-19: from immunity to prognosis, Avicenna J Med Biotechnol, doi:10.18502/ajmb.v15i2.12022
Black, Molecular modeling and preliminary clinical data suggesting antiviral activity for chlorpheniramine (chlorphenamine) against COVID-19, Cureus, doi:10.7759/cureus.20980
Blanco, Bonilla, Smith, Gómez De, Heras, Antihistamines as an early treatment for Covid-19, Heliyon, doi:10.1016/j.heliyon.2023.e15772
Chaudhari, Gosavi, Bornare, An overview of antihistamines and their properties used for treatment of different diseases, Antiinflamm Antiallergy Agents Med Chem, doi:10.2174/0118715230259623231111165759
Conaway S Jr, Huang, Hernandez-Lara, Molecular mechanism of bitter taste receptor agonistmediated relaxation of airway smooth muscle, FASEB J, doi:10.1096/fj.202400452R
Elshaier, Mostafa, Valerio-Pascua, Chlorpheniramine maleate displays multiple modes of antiviral action against SARS-CoV-2: a mechanistic study, bioRxiv, doi:10.2139/ssrn.4603627
Ennis, Tiligada, Histamine receptors and COVID-19, Inflamm Res, doi:10.1007/s00011-020-01422-1
Gamage, Tan, Chan, Human nasal epithelial cells sustain persistent SARS-CoV-2 infection in vitro, despite eliciting a prolonged antiviral response, mBio, doi:10.1128/mbio.03436-21
Ge, Wang, Hou, Repositioning of histamine H(1) receptor antagonist: Doxepin inhibits viropexis of SARS-CoV-2 Spike pseudovirus by blocking ACE2, Eur J Pharmacol, doi:10.1016/j.ejphar.2021.173897
Ghzaiel, Sassi, Zarrouk, 7-Ketocholesterol: Effects on viral infections and hypothetical contribution in COVID-19, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2021.105939
Ii, Iii, Cannon, Dual-histamine receptor blockade with cetirizine -famotidine reduces pulmonary symptoms in COVID-19 patients, Pulm Pharmacol Ther, doi:10.1016/j.pupt.2020.101942
Kumar, Cheng, A hypothesis: Bitter taste receptors as a therapeutic target for the clinical symptoms of SARS-CoV-2, Pharmazie, doi:10.1691/ph.2021.0840
Kumar, Kumar, Dsouza, Bitter taste receptors establish a stable binding affinity with the SARS-CoV-2-spike 1 protein akin to ACE2, J Biomol Struct Dyn, doi:10.1080/07391102.2023.2300128
Kusiak, Cichońska, Tubaja, COVID-19 manifestation in the oral cavity -a narrative literature review, Acta Otorhinolaryngol Ital, doi:10.14639/0392-100X-N1584
Li, Zhang, Liu, Gu, Existing bitter medicines for fighting 2019-nCoV-associated infectious diseases, FASEB J, doi:10.1096/fj.202000502
Ma, Li, Pan, Phillyrin (KD-1) exerts anti-viral and anti-inflammatory activities against novel coronavirus (SARS-CoV-2) and human coronavirus 229E (HCoV-229E) by suppressing the nuclear factor kappa B (NF-κB) signaling pathway, Phytomedicine, doi:10.1016/j.phymed.2020.153296
Malone, Tisdall, Smith, COVID-19: Famotidine, histamine, mast cells, and mechanisms, Front Pharmacol, doi:10.3389/fphar.2021.633680
Qu, Fuhler, Pan, Could histamine H1 receptor antagonists be used for treating COVID-19?, Int J Mol Sci, doi:10.3390/ijms22115672
Risso, Carmagnola, Morini, Distribution of TAS2R38 bitter taste receptor phenotype and haplotypes among COVID-19 patients, Sci Rep, doi:10.1038/s41598-022-10747-2
Rizvi, Ferrer, Khawaja, Sanchez-Gonzalez, Chlorpheniramine, an old drug with new potential clinical applications: a comprehensive review of the literature, Curr Rev Clin Exp Pharmacol, doi:10.2174/2772432817666220601162006
Roumestan, Henriquet, Gougat, Histamine H1-receptor antagonists inhibit nuclear factor-kappaB and activator protein-1 activities via H1-receptor-dependent and -independent mechanisms, Clin Exp Allergy, doi:10.1111/j.1365-2222.2008.02990.x
Salvucci, Codella, Coppola, Antihistamines improve cardiovascular manifestations and other symptoms of long-COVID attributed to mast cell activation, Front Cardiovasc Med, doi:10.3389/fcvm.2023.1202696
Sanchez-Gonzalez, Westover, Rizvi, Intranasal chlorpheniramine maleate for the treatment of COVID-19: translational and clinical evidence, Med Res Arch, doi:10.10.18103/mra.v10i3.2752
Santin, Spedicati, Pecori, The bittersweet symphony of COVID-19: associations between TAS1Rs and TAS2R38 genetic variations and COVID-19 symptoms, Life, doi:10.3390/life14020219
Thangam, Jemima, Singh, The role of histamine and histamine receptors in mast cellmediated allergy and inflammation: the hunt for new therapeutic targets, Front Immunol, doi:10.3389/fimmu.2018.01873
Valerio-Pascua, Baires, Sekhon, Mitigating the risks of post-acute sequelae of SARS-CoV-2 infection (PASC) with intranasal chlorpheniramine: perspectives from the ACCROS studies, BMC Infect Dis, doi:10.1186/s12879-024-10211-8
Valerio-Pascua, Mejia, Tesch, Chlorpheniramine intranasal spray to accelerate COVID-19 clinical recovery in an outpatient setting: The ACCROS trials, Res Squ, doi:10.21203/rs.3.rs-2167465/v1
Wang, Guo, Wu, Molecular mechanism of antihistamines recognition and regulation of the histamine receptor, Nat Commun, doi:10.1038/s41467-023-44477-4
Westover, Ferrer, Vazquez, In vitro virucidal effect of intranasally delivered chlorpheniramine maleate compound against severe acute respiratory syndrome coronavirus 2, Cureus, doi:10.7759/cureus.10501
Yu, Li, Xia, Tang, Expression and localization of histamine H(1), H(2), and H(3) receptors in rat olfactory epithelium, Int J Pediatr Otorhinolaryngol, doi:10.1016/j.ijporl.2017.07.045
Yu, Liu, Ou, The histamine receptor H1 acts as an alternative receptor for SARS-CoV-2, mBio, doi:10.1128/mbio.01088-24
DOI record:
{
"DOI": "10.7759/cureus.82736",
"ISSN": [
"2168-8184"
],
"URL": "http://dx.doi.org/10.7759/cureus.82736",
"author": [
{
"affiliation": [],
"family": "Ferrer",
"given": "Gustavo",
"sequence": "first"
},
{
"affiliation": [],
"family": "Valerio-Pascua",
"given": "Fernando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alas-Pineda",
"given": "César",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gaitán-Zambrano",
"given": "Kristhel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pavón-Varela",
"given": "Dennis J",
"sequence": "additional"
}
],
"container-title": "Cureus",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
4,
21
]
],
"date-time": "2025-04-21T23:06:21Z",
"timestamp": 1745276781000
},
"deposited": {
"date-parts": [
[
2025,
4,
21
]
],
"date-time": "2025-04-21T23:06:22Z",
"timestamp": 1745276782000
},
"indexed": {
"date-parts": [
[
2025,
4,
22
]
],
"date-time": "2025-04-22T04:05:27Z",
"timestamp": 1745294727966,
"version": "3.40.4"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
4,
21
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.cureus.com/articles/350279-intranasal-chlorpheniramine-for-early-symptomatic-treatment-of-covid-19-and-the-impact-on-long-covid",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.7759",
"published": {
"date-parts": [
[
2025,
4,
21
]
]
},
"published-print": {
"date-parts": [
[
2025,
4,
21
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.3389/fphar.2021.633680",
"article-title": "COVID-19: Famotidine, histamine, mast cells, and mechanisms",
"author": "Malone RW",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol",
"key": "ref1",
"unstructured": "Malone RW, Tisdall P, Fremont-Smith P, et al.. COVID-19: Famotidine, histamine, mast cells, and mechanisms. Front Pharmacol. 2021, 12:633680. 10.3389/fphar.2021.633680",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3389/fcvm.2023.1202696",
"article-title": "Antihistamines improve cardiovascular manifestations and other symptoms of long-COVID attributed to mast cell activation",
"author": "Salvucci F",
"doi-asserted-by": "publisher",
"journal-title": "Front Cardiovasc Med",
"key": "ref2",
"unstructured": "Salvucci F, Codella R, Coppola A, et al.. Antihistamines improve cardiovascular manifestations and other symptoms of long-COVID attributed to mast cell activation. Front Cardiovasc Med. 2023, 10:1202696. 10.3389/fcvm.2023.1202696",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.3390/ijms22115672",
"article-title": "Could histamine H1 receptor antagonists be used for treating COVID-19?",
"author": "Qu C",
"doi-asserted-by": "publisher",
"journal-title": "Int J Mol Sci",
"key": "ref3",
"unstructured": "Qu C, Fuhler GM, Pan Y. Could histamine H1 receptor antagonists be used for treating COVID-19?. Int J Mol Sci. 2021, 22:10.3390/ijms22115672",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1128/mbio.01088-24",
"article-title": "The histamine receptor H1 acts as an alternative receptor for SARS-CoV-2",
"author": "Yu F",
"doi-asserted-by": "publisher",
"journal-title": "mBio",
"key": "ref4",
"unstructured": "Yu F, Liu X, Ou H, et al.. The histamine receptor H1 acts as an alternative receptor for SARS-CoV-2. mBio. 2024, 15:e0108824. 10.1128/mbio.01088-24",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.3390/life14020219",
"article-title": "The bittersweet symphony of COVID-19: associations between TAS1Rs and TAS2R38 genetic variations and COVID-19 symptoms",
"author": "Santin A",
"doi-asserted-by": "publisher",
"journal-title": "Life (Basel)",
"key": "ref5",
"unstructured": "Santin A, Spedicati B, Pecori A, et al.. The bittersweet symphony of COVID-19: associations between TAS1Rs and TAS2R38 genetic variations and COVID-19 symptoms. Life (Basel). 2024, 14:10.3390/life14020219",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.1038/s41598-022-10747-2",
"article-title": "Distribution of TAS2R38 bitter taste receptor phenotype and haplotypes among COVID-19 patients",
"author": "Risso D",
"doi-asserted-by": "publisher",
"journal-title": "Sci Rep",
"key": "ref6",
"unstructured": "Risso D, Carmagnola D, Morini G, et al.. Distribution of TAS2R38 bitter taste receptor phenotype and haplotypes among COVID-19 patients. Sci Rep. 2022, 12:7381. 10.1038/s41598-022-10747-2",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1007/s00011-020-01422-1",
"article-title": "Histamine receptors and COVID-19",
"author": "Ennis M",
"doi-asserted-by": "publisher",
"journal-title": "Inflamm Res",
"key": "ref7",
"unstructured": "Ennis M, Tiligada K. Histamine receptors and COVID-19. Inflamm Res. 2021, 70:67-75. 10.1007/s00011-020-01422-1",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1096/fj.202000502",
"article-title": "Existing bitter medicines for fighting 2019-nCoV-associated infectious diseases",
"author": "Li X",
"doi-asserted-by": "publisher",
"journal-title": "FASEB J",
"key": "ref8",
"unstructured": "Li X, Zhang C, Liu L, Gu M. Existing bitter medicines for fighting 2019-nCoV-associated infectious diseases. FASEB J. 2020, 34:6008-16. 10.1096/fj.202000502",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1186/s12879-024-10211-8",
"article-title": "Mitigating the risks of post-acute sequelae of SARS-CoV-2 infection (PASC) with intranasal chlorpheniramine: perspectives from the ACCROS studies",
"author": "Valerio-Pascua F",
"doi-asserted-by": "publisher",
"journal-title": "BMC Infect Dis",
"key": "ref9",
"unstructured": "Valerio-Pascua F, Baires F, Sekhon AK, et al.. Mitigating the risks of post-acute sequelae of SARS-CoV-2 infection (PASC) with intranasal chlorpheniramine: perspectives from the ACCROS studies. BMC Infect Dis. 2024, 24:1348. 10.1186/s12879-024-10211-8",
"volume": "24",
"year": "2024"
},
{
"DOI": "10.1038/s41467-023-44477-4",
"article-title": "Molecular mechanism of antihistamines recognition and regulation of the histamine H(1) receptor",
"author": "Wang D",
"doi-asserted-by": "publisher",
"journal-title": "Nat Commun",
"key": "ref10",
"unstructured": "Wang D, Guo Q, Wu Z, et al.. Molecular mechanism of antihistamines recognition and regulation of the histamine H(1) receptor. Nat Commun. 2024, 15:84. 10.1038/s41467-023-44477-4",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1016/j.heliyon.2023.e15772",
"article-title": "Antihistamines as an early treatment for Covid-19",
"author": "Morán Blanco JI",
"doi-asserted-by": "publisher",
"journal-title": "Heliyon",
"key": "ref11",
"unstructured": "Morán Blanco JI, Alvarenga Bonilla JA, Fremont-Smith P, Villar Gómez de Las Heras K. Antihistamines as an early treatment for Covid-19. Heliyon. 2023, 9:e15772. 10.1016/j.heliyon.2023.e15772",
"volume": "9",
"year": "2023"
},
{
"DOI": "10.2174/0118715230259623231111165759",
"article-title": "An overview of antihistamines and their properties used for treatment of different diseases",
"author": "Chaudhari R",
"doi-asserted-by": "publisher",
"journal-title": "Antiinflamm Antiallergy Agents Med Chem",
"key": "ref12",
"unstructured": "Chaudhari R, Gosavi S, Bornare P, et al.. An overview of antihistamines and their properties used for treatment of different diseases. Antiinflamm Antiallergy Agents Med Chem. 2023, 22:220-9. 10.2174/0118715230259623231111165759",
"volume": "22",
"year": "2023"
},
{
"DOI": "10.1016/j.ijporl.2017.07.045",
"article-title": "Expression and localization of histamine H(1), H(2), and H(3) receptors in rat olfactory epithelium",
"author": "Yu C",
"doi-asserted-by": "publisher",
"journal-title": "Int J Pediatr Otorhinolaryngol",
"key": "ref13",
"unstructured": "Yu C, Li L, Xia Q, Tang Y. Expression and localization of histamine H(1), H(2), and H(3) receptors in rat olfactory epithelium. Int J Pediatr Otorhinolaryngol. 2017, 101:102-6. 10.1016/j.ijporl.2017.07.045",
"volume": "101",
"year": "2017"
},
{
"DOI": "10.1096/fj.202400452R",
"article-title": "Molecular mechanism of bitter taste receptor agonist-mediated relaxation of airway smooth muscle",
"author": "Conaway S Jr",
"doi-asserted-by": "publisher",
"journal-title": "FASEB J",
"key": "ref14",
"unstructured": "Conaway S Jr, Huang W, Hernandez-Lara MA, et al.. Molecular mechanism of bitter taste receptor agonist-mediated relaxation of airway smooth muscle. FASEB J. 2024, 38:e23842. 10.1096/fj.202400452R",
"volume": "38",
"year": "2024"
},
{
"DOI": "10.1016/j.jsbmb.2021.105939",
"article-title": "7-Ketocholesterol: Effects on viral infections and hypothetical contribution in COVID-19",
"author": "Ghzaiel I",
"doi-asserted-by": "publisher",
"journal-title": "J Steroid Biochem Mol Biol",
"key": "ref15",
"unstructured": "Ghzaiel I, Sassi K, Zarrouk A, et al.. 7-Ketocholesterol: Effects on viral infections and hypothetical contribution in COVID-19. J Steroid Biochem Mol Biol. 2021, 212:105939. 10.1016/j.jsbmb.2021.105939",
"volume": "212",
"year": "2021"
},
{
"DOI": "10.14639/0392-100X-N1584",
"article-title": "COVID-19 manifestation in the oral cavity - a narrative literature review",
"author": "Kusiak A",
"doi-asserted-by": "publisher",
"journal-title": "Acta Otorhinolaryngol Ital",
"key": "ref16",
"unstructured": "Kusiak A, Cichońska D, Tubaja M, et al.. COVID-19 manifestation in the oral cavity - a narrative literature review. Acta Otorhinolaryngol Ital. 2021, 41:395-400. 10.14639/0392-100X-N1584",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1128/mbio.03436-21",
"article-title": "Human nasal epithelial cells sustain persistent SARS-CoV-2 infection in vitro, despite eliciting a prolonged antiviral response",
"author": "Gamage AM",
"doi-asserted-by": "publisher",
"journal-title": "mBio",
"key": "ref17",
"unstructured": "Gamage AM, Tan KS, Chan WO, et al.. Human nasal epithelial cells sustain persistent SARS-CoV-2 infection in vitro, despite eliciting a prolonged antiviral response. mBio. 2022, 13:e0343621. 10.1128/mbio.03436-21",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2018.01873",
"article-title": "The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets",
"author": "Thangam EB",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "ref18",
"unstructured": "Thangam EB, Jemima EA, Singh H, et al.. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Front Immunol. 2018, 9:1873. 10.3389/fimmu.2018.01873",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1016/j.phymed.2020.153296",
"article-title": "Phillyrin (KD-1) exerts anti-viral and anti-inflammatory activities against novel coronavirus (SARS-CoV-2) and human coronavirus 229E (HCoV-229E) by suppressing the nuclear factor kappa B (NF-κB) signaling pathway",
"author": "Ma Q",
"doi-asserted-by": "publisher",
"journal-title": "Phytomedicine",
"key": "ref19",
"unstructured": "Ma Q, Li R, Pan W, et al.. Phillyrin (KD-1) exerts anti-viral and anti-inflammatory activities against novel coronavirus (SARS-CoV-2) and human coronavirus 229E (HCoV-229E) by suppressing the nuclear factor kappa B (NF-κB) signaling pathway. Phytomedicine. 2020, 78:153296. 10.1016/j.phymed.2020.153296",
"volume": "78",
"year": "2020"
},
{
"DOI": "10.1111/j.1365-2222.2008.02990.x",
"article-title": "Histamine H1-receptor antagonists inhibit nuclear factor-kappaB and activator protein-1 activities via H1-receptor-dependent and -independent mechanisms",
"author": "Roumestan C",
"doi-asserted-by": "publisher",
"journal-title": "Clin Exp Allergy",
"key": "ref20",
"unstructured": "Roumestan C, Henriquet C, Gougat C, et al.. Histamine H1-receptor antagonists inhibit nuclear factor-kappaB and activator protein-1 activities via H1-receptor-dependent and -independent mechanisms. Clin Exp Allergy. 2008, 38:947-56. 10.1111/j.1365-2222.2008.02990.x",
"volume": "38",
"year": "2008"
},
{
"DOI": "10.1016/j.ejphar.2021.173897",
"article-title": "Repositioning of histamine H(1) receptor antagonist: Doxepin inhibits viropexis of SARS-CoV-2 Spike pseudovirus by blocking ACE2",
"author": "Ge S",
"doi-asserted-by": "publisher",
"journal-title": "Eur J Pharmacol",
"key": "ref21",
"unstructured": "Ge S, Wang X, Hou Y, et al.. Repositioning of histamine H(1) receptor antagonist: Doxepin inhibits viropexis of SARS-CoV-2 Spike pseudovirus by blocking ACE2. Eur J Pharmacol. 2021, 896:173897. 10.1016/j.ejphar.2021.173897",
"volume": "896",
"year": "2021"
},
{
"DOI": "10.1016/j.pupt.2020.101942",
"article-title": "Dual-histamine receptor blockade with cetirizine - famotidine reduces pulmonary symptoms in COVID-19 patients",
"author": "Hogan Ii RB",
"doi-asserted-by": "publisher",
"journal-title": "Pulm Pharmacol Ther",
"key": "ref22",
"unstructured": "Hogan Ii RB, Hogan Iii RB, Cannon T, et al.. Dual-histamine receptor blockade with cetirizine - famotidine reduces pulmonary symptoms in COVID-19 patients. Pulm Pharmacol Ther. 2020, 63:101942. 10.1016/j.pupt.2020.101942",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.7759/cureus.20980",
"article-title": "Molecular modeling and preliminary clinical data suggesting antiviral activity for chlorpheniramine (chlorphenamine) against COVID-19",
"author": "Black SD",
"doi-asserted-by": "publisher",
"journal-title": "Cureus",
"key": "ref23",
"unstructured": "Black SD. Molecular modeling and preliminary clinical data suggesting antiviral activity for chlorpheniramine (chlorphenamine) against COVID-19. Cureus. 2022, 14:e20980. 10.7759/cureus.20980",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.2174/2772432817666220601162006",
"article-title": "Chlorpheniramine, an old drug with new potential clinical applications: a comprehensive review of the literature",
"author": "Rizvi SA",
"doi-asserted-by": "publisher",
"journal-title": "Curr Rev Clin Exp Pharmacol",
"key": "ref24",
"unstructured": "Rizvi SA, Ferrer G, Khawaja UA, Sanchez-Gonzalez MA. Chlorpheniramine, an old drug with new potential clinical applications: a comprehensive review of the literature. Curr Rev Clin Exp Pharmacol. 2024, 19:137-45. 10.2174/2772432817666220601162006",
"volume": "19",
"year": "2024"
},
{
"DOI": "10.18502/ajmb.v15i2.12022",
"article-title": "Human T2R38 bitter taste receptor expression and COVID- 19: from immunity to prognosis",
"author": "Bethineedi LD",
"doi-asserted-by": "publisher",
"journal-title": "Avicenna J Med Biotechnol",
"key": "ref25",
"unstructured": "Bethineedi LD, Baghsheikhi H, Soltani A, et al.. Human T2R38 bitter taste receptor expression and COVID- 19: from immunity to prognosis. Avicenna J Med Biotechnol. 2023, 15:118-23. 10.18502/ajmb.v15i2.12022",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1080/07391102.2023.2300128",
"article-title": "Bitter taste receptors establish a stable binding affinity with the SARS-CoV-2-spike 1 protein akin to ACE2",
"author": "Kumar SA",
"doi-asserted-by": "publisher",
"journal-title": "J Biomol Struct Dyn",
"key": "ref26",
"unstructured": "Kumar SA, Selvaa Kumar C, Dsouza N. Bitter taste receptors establish a stable binding affinity with the SARS-CoV-2-spike 1 protein akin to ACE2. J Biomol Struct Dyn. 2025, 43:3845-58. 10.1080/07391102.2023.2300128",
"volume": "43",
"year": "2025"
},
{
"DOI": "10.1691/ph.2021.0840",
"article-title": "A hypothesis: Bitter taste receptors as a therapeutic target for the clinical symptoms of SARS-CoV-2",
"author": "Kumar SA",
"doi-asserted-by": "publisher",
"journal-title": "Pharmazie",
"key": "ref27",
"unstructured": "Kumar SA, Cheng W. A hypothesis: Bitter taste receptors as a therapeutic target for the clinical symptoms of SARS-CoV-2. Pharmazie. 2021, 76:43-54. 10.1691/ph.2021.0840",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1002/alr.22692",
"article-title": "Does phenotypic expression of bitter taste receptor T2R38 show association with COVID-19 severity?",
"author": "Barham HP",
"doi-asserted-by": "publisher",
"journal-title": "Int Forum Allergy Rhinol",
"key": "ref28",
"unstructured": "Barham HP, Taha MA, Hall CA. Does phenotypic expression of bitter taste receptor T2R38 show association with COVID-19 severity?. Int Forum Allergy Rhinol. 2020, 10:1255-7. 10.1002/alr.22692",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.2139/ssrn.4603627",
"article-title": "Chlorpheniramine maleate displays multiple modes of antiviral action against SARS-CoV-2: a mechanistic study",
"author": "Elshaier Y",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv",
"key": "ref29",
"unstructured": "Elshaier Y, Mostafa A, Valerio-Pascua F, et al.. Chlorpheniramine maleate displays multiple modes of antiviral action against SARS-CoV-2: a mechanistic study. bioRxiv. 2023, 10.2139/ssrn.4603627",
"year": "2023"
},
{
"DOI": "10. 10.18103/mra.v10i3.2752",
"article-title": "Intranasal chlorpheniramine maleate for the treatment of COVID- 19: translational and clinical evidence",
"author": "Sanchez-Gonzalez M",
"doi-asserted-by": "publisher",
"journal-title": "Med Res Arch",
"key": "ref30",
"unstructured": "Sanchez-Gonzalez M, Westover J, Rizvi S, et al.. Intranasal chlorpheniramine maleate for the treatment of COVID- 19: translational and clinical evidence. Med Res Arch. 2022, 10. 10.18103/mra.v10i3.2752",
"year": "2022"
},
{
"DOI": "10.21203/rs.3.rs-2167465/v1",
"article-title": "Chlorpheniramine intranasal spray to accelerate COVID-19 clinical recovery in an outpatient setting: The ACCROS trials",
"author": "Valerio-Pascua F",
"doi-asserted-by": "publisher",
"journal-title": "Res Squ",
"key": "ref31",
"unstructured": "Valerio-Pascua F, Mejia EJP, Tesch ML, et al.. Chlorpheniramine intranasal spray to accelerate COVID-19 clinical recovery in an outpatient setting: The ACCROS trials. Res Squ. 2022, 10.21203/rs.3.rs-2167465/v1",
"year": "2022"
},
{
"DOI": "10.7759/cureus.10501",
"article-title": "In vitro virucidal effect of intranasally delivered chlorpheniramine maleate compound against severe acute respiratory syndrome coronavirus 2",
"author": "Westover JB",
"doi-asserted-by": "publisher",
"journal-title": "Cureus",
"key": "ref32",
"unstructured": "Westover JB, Ferrer G, Vazquez H, et al.. In vitro virucidal effect of intranasally delivered chlorpheniramine maleate compound against severe acute respiratory syndrome coronavirus 2. Cureus. 2020, 12:e10501. 10.7759/cureus.10501",
"volume": "12",
"year": "2020"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.cureus.com/articles/350279-intranasal-chlorpheniramine-for-early-symptomatic-treatment-of-covid-19-and-the-impact-on-long-covid"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Intranasal Chlorpheniramine for Early Symptomatic Treatment of COVID-19 and the Impact on Long-COVID",
"type": "journal-article"
}
