Colchicine and aspirin in community patients with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial
et al., The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(22)00299-5, ACT outpatient, NCT04324463, Oct 2022
Late (5.4 days) outpatient RCT showing no significant difference in outcomes with aspirin treatment.
Study covers aspirin and colchicine.
|
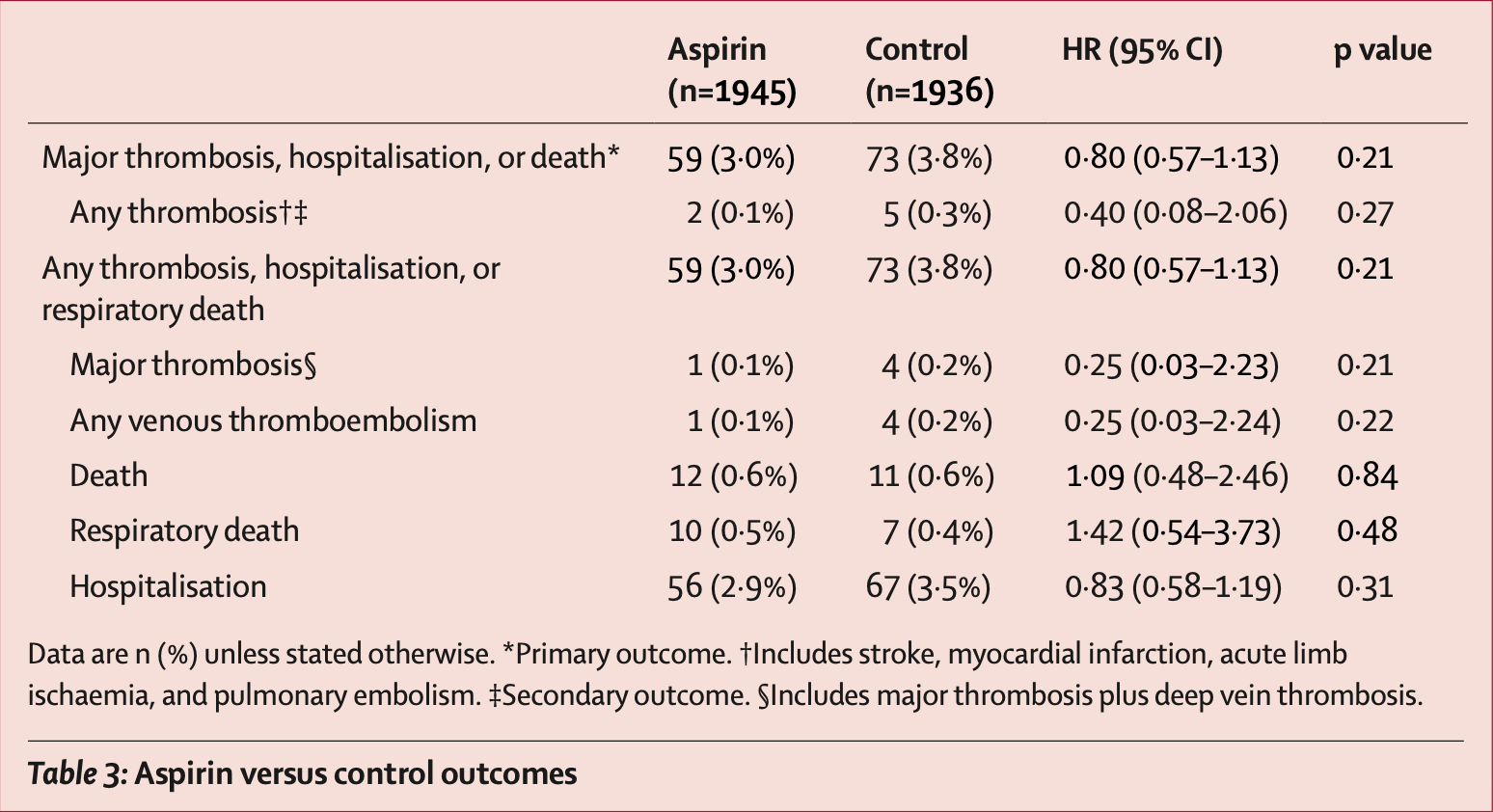
risk of death, 9.0% higher, HR 1.09, p = 0.84, treatment 12 of 1,945 (0.6%), control 11 of 1,936 (0.6%).
|
|
risk of progression, 20.0% lower, HR 0.80, p = 0.21, treatment 59 of 1,945 (3.0%), control 73 of 1,936 (3.8%), NNT 136, major thrombosis, hospitalisation, or death, primary outcome.
|
|
risk of hospitalization, 17.0% lower, HR 0.83, p = 0.31, treatment 56 of 1,945 (2.9%), control 67 of 1,936 (3.5%), NNT 172.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Eikelboom et al., 10 Oct 2022, Randomized Controlled Trial, Canada, peer-reviewed, mean age 45.0, 31 authors, study period 27 August, 2020 - 10 February, 2022, average treatment delay 5.4 days, trial NCT04324463 (history) (ACT outpatient).
Contact: eikelbj@mcmaster.ca.
Colchicine and aspirin in community patients with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial
The Lancet Respiratory Medicine, doi:10.1016/s2213-2600(22)00299-5
Background The large number of patients worldwide infected with the SARS-CoV-2 virus has overwhelmed healthcare systems globally. The Anti-Coronavirus Therapies (ACT) outpatient trial aimed to evaluate anti-inflammatory therapy with colchicine and antithrombotic therapy with aspirin for prevention of disease progression in community patients with COVID-19.
Methods The ACT outpatient, open-label, 2 × 2 factorial, randomised, controlled trial, was done at 48 clinical sites in 11 countries. Patients in the community aged 30 years and older with symptomatic, laboratory confirmed COVID-19 who were within 7 days of diagnosis and at high risk of disease progression were randomly assigned (1:1) to receive colchicine 0•6 mg twice daily for 3 days and then 0•6 mg once daily for 25 days versus usual care, and in a second (1:1) randomisation to receive aspirin 100 mg once daily for 28 days versus usual care. Investigators and patients were not masked to treatment allocation. The primary outcome was assessed at 45 days in the intention-to-treat population; for the colchicine randomisation it was hospitalisation or death, and for the aspirin randomisation it was major thrombosis, hospitalisation, or death. The ACT outpatient trial is registered at ClinicalTrials.gov, NCT04324463 and is ongoing.
References
Ananworanich, Mogg, Dunne, Randomized study of rivaroxaban vs. placebo on disease progression and symptoms resolution in high-risk adults with mild COVID-19, Clin Infect Dis
Baigent, Blackwell, Collins, Aspirin in the primary and secondary prevention of vascular disease: collaborative metaanalysis of individual participant data from randomised trials, Lancet
Baker, Mahdi, Nicolau, Jr, Early Th2 inflammation in the upper respiratory mucosa as a predictor of severe COVID-19 and modulation by early treatment with inhaled corticosteroids: a mechanistic analysis, Lancet Respir Med
Barco, Voci, Held, Enoxaparin for primary thromboprophylaxis in symptomatic outpatients with COVID-19 (OVID): a randomised, open-label, parallel-group, multicentre, phase 3 trial, Lancet Haematol
Bollyky, Nuzzo, Huhn, Kiernan, Pond, Global vaccination must be swifter, Nature
Bonaventura, Vecchié, Dagna, Tangianu, Abbate et al., Colchicine for COVID-19: targeting NLRP3 inflammasome to blunt hyperinflammation, Inflamm Res
Connors, Brooks, Sciurba, Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: The ACTIV-4B randomized clinical trial, JAMA
Cools, Virdone, Sawhney, Thromboprophylactic lowmolecular-weight heparin versus standard of care in unvaccinated, at-risk outpatients with COVID-19 (ETHIC): an open-label, multicentre, randomised, controlled, phase 3b trial, Lancet Haematol
Covid-, Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21, Lancet
Deftereos, Giannopoulos, Vrachatis, Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: The GRECCO-19 Randomized Clinical Trial, JAMA Netw Open
Diaz, Orlandini, Castellana, Effect of colchicine vs usual care alone on intubation and 28-day mortality in patients hospitalized with COVID-19: a randomized clinical trial, JAMA Netw Open
Eikelboom, Jolly, Belley-Cote, Colchicine and the combination of rivaroxaban and aspirin in patients hospitalised with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(22)00298-3
Eikelboom, Kearon, Guyatt, Perioperative aspirin for prevention of venous thromboembolism: the perioperative ischemia evaluation-2 trial and a pooled analysis of the randomized trials, Anesthesiology
Eikelboom, Rangarajan, Jolly, The Anti-Coronavirus Therapies (ACT) trials: design, baseline characteristics, and challenges, CJC Open
Gorial, Maulood, Abdulamir, Alnuaimi, Abdulrrazaq et al., Randomized controlled trial of colchicine add on to the standard therapy in moderate and severe corona virus Disease-19 infection, Ann Med Surg (Lond)
Group, Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet Respir Med
Leentjens, Van Haaps, Wessels, Schutgens, Middeldorp, COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year, Lancet Haematol
Osuchowski, Winkler, Skirecki, The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity, Lancet Respir Med
Pavia, Pasteur, vaccines, and the refusal to become fully vaccinated in the midst of the COVID-19 pandemic, Front Public Health
Pogue, Walter, Yusuf, Evaluating the benefit of event adjudication of cardiovascular outcomes in large simple RCTs, Clin Trials
Simes, Becattini, Agnelli, Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration, Circulation
Tardif, Bouabdallaoui, Allier, Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebocontrolled, multicentre trial, Lancet Respir Med
DOI record:
{
"DOI": "10.1016/s2213-2600(22)00299-5",
"ISSN": [
"2213-2600"
],
"URL": "http://dx.doi.org/10.1016/S2213-2600(22)00299-5",
"alternative-id": [
"S2213260022002995"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Colchicine and aspirin in community patients with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Respiratory Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S2213-2600(22)00299-5"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S2213-2600(22)00368-X"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Eikelboom",
"given": "John W",
"sequence": "first"
},
{
"affiliation": [],
"family": "Jolly",
"given": "Sanjit S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Belley-Cote",
"given": "Emilie P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Whitlock",
"given": "Richard P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rangarajan",
"given": "Sumathy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Lizhen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heenan",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bangdiwala",
"given": "Shrikant I",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tarhuni",
"given": "Wadea M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hassany",
"given": "Mohamed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kontsevaya",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harper",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharma",
"given": "Sanjib Kumar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lopez-Jaramillo",
"given": "Patricio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dans",
"given": "Antonio L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Palileo-Villanueva",
"given": "Lia M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Avezum",
"given": "Alvaro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pais",
"given": "Prem",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xavier",
"given": "Denis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Felix",
"given": "Camilo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yusufali",
"given": "Afzalhussein",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lopes",
"given": "Renato D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berwanger",
"given": "Otavio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ali",
"given": "Zeeshan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wasserman",
"given": "Sean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anand",
"given": "Sonia S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bosch",
"given": "Jackie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choudhri",
"given": "Shurjeel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farkouh",
"given": "Michael E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Loeb",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yusuf",
"given": "Salim",
"sequence": "additional"
}
],
"container-title": "The Lancet Respiratory Medicine",
"container-title-short": "The Lancet Respiratory Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
10,
10
]
],
"date-time": "2022-10-10T22:31:57Z",
"timestamp": 1665441117000
},
"deposited": {
"date-parts": [
[
2022,
10,
10
]
],
"date-time": "2022-10-10T22:32:18Z",
"timestamp": 1665441138000
},
"indexed": {
"date-parts": [
[
2022,
10,
10
]
],
"date-time": "2022-10-10T23:11:27Z",
"timestamp": 1665443487968
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
1
]
],
"date-time": "2022-10-01T00:00:00Z",
"timestamp": 1664582400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260022002995?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260022002995?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
10
]
]
},
"published-print": {
"date-parts": [
[
2022,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/S0140-6736(22)00484-6",
"article-title": "Estimating global, regional, and national daily and cumulative infections with SARS-CoV-2 through Nov 14, 2021: a statistical analysis",
"doi-asserted-by": "crossref",
"first-page": "2351",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(22)00299-5_bib1",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)02796-3",
"article-title": "Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21",
"doi-asserted-by": "crossref",
"first-page": "1513",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(22)00299-5_bib2",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1038/d41586-022-00809-w",
"article-title": "Global vaccination must be swifter",
"author": "Bollyky",
"doi-asserted-by": "crossref",
"first-page": "788",
"journal-title": "Nature",
"key": "10.1016/S2213-2600(22)00299-5_bib3",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.3389/fpubh.2022.815816",
"article-title": "Pasteur, vaccines, and the refusal to become fully vaccinated in the midst of the COVID-19 pandemic",
"author": "Pavia",
"doi-asserted-by": "crossref",
"journal-title": "Front Public Health",
"key": "10.1016/S2213-2600(22)00299-5_bib4",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(21)00218-6",
"article-title": "The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity",
"author": "Osuchowski",
"doi-asserted-by": "crossref",
"first-page": "622",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(22)00299-5_bib5",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(22)00002-9",
"article-title": "Early Th2 inflammation in the upper respiratory mucosa as a predictor of severe COVID-19 and modulation by early treatment with inhaled corticosteroids: a mechanistic analysis",
"author": "Baker",
"doi-asserted-by": "crossref",
"first-page": "545",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(22)00299-5_bib6",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1007/s00011-022-01540-y",
"article-title": "Colchicine for COVID-19: targeting NLRP3 inflammasome to blunt hyperinflammation",
"author": "Bonaventura",
"doi-asserted-by": "crossref",
"first-page": "293",
"journal-title": "Inflamm Res",
"key": "10.1016/S2213-2600(22)00299-5_bib7",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(09)60503-1",
"article-title": "Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials",
"author": "Baigent",
"doi-asserted-by": "crossref",
"first-page": "1849",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(22)00299-5_bib8",
"volume": "373",
"year": "2009"
},
{
"DOI": "10.1161/CIRCULATIONAHA.114.008828",
"article-title": "Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration",
"author": "Simes",
"doi-asserted-by": "crossref",
"first-page": "1062",
"journal-title": "Circulation",
"key": "10.1016/S2213-2600(22)00299-5_bib9",
"volume": "130",
"year": "2014"
},
{
"DOI": "10.1097/ALN.0000000000001352",
"article-title": "Perioperative aspirin for prevention of venous thromboembolism: the perioperative ischemia evaluation-2 trial and a pooled analysis of the randomized trials",
"author": "Eikelboom",
"doi-asserted-by": "crossref",
"first-page": "1121",
"journal-title": "Anesthesiology",
"key": "10.1016/S2213-2600(22)00299-5_bib10",
"volume": "125",
"year": "2016"
},
{
"DOI": "10.1016/S2213-2600(21)00222-8",
"article-title": "Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial",
"author": "Tardif",
"doi-asserted-by": "crossref",
"first-page": "924",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(22)00299-5_bib11",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.17272",
"article-title": "Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: The ACTIV-4B randomized clinical trial",
"author": "Connors",
"doi-asserted-by": "crossref",
"first-page": "1703",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(22)00299-5_bib12",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciab813",
"article-title": "Randomized study of rivaroxaban vs. placebo on disease progression and symptoms resolution in high-risk adults with mild COVID-19",
"author": "Ananworanich",
"doi-asserted-by": "crossref",
"first-page": "e473",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S2213-2600(22)00299-5_bib13",
"volume": "75",
"year": "2021"
},
{
"DOI": "10.1016/S2352-3026(22)00173-9",
"article-title": "Thromboprophylactic low-molecular-weight heparin versus standard of care in unvaccinated, at-risk outpatients with COVID-19 (ETHIC): an open-label, multicentre, randomised, controlled, phase 3b trial",
"author": "Cools",
"doi-asserted-by": "crossref",
"first-page": "e594",
"journal-title": "Lancet Haematol",
"key": "10.1016/S2213-2600(22)00299-5_bib14",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1016/S2352-3026(22)00175-2",
"article-title": "Enoxaparin for primary thromboprophylaxis in symptomatic outpatients with COVID-19 (OVID): a randomised, open-label, parallel-group, multicentre, phase 3 trial",
"author": "Barco",
"doi-asserted-by": "crossref",
"first-page": "e585",
"journal-title": "Lancet Haematol",
"key": "10.1016/S2213-2600(22)00299-5_bib15",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1016/j.cjco.2022.02.010",
"article-title": "The Anti-Coronavirus Therapies (ACT) trials: design, baseline characteristics, and challenges",
"author": "Eikelboom",
"doi-asserted-by": "crossref",
"first-page": "568",
"journal-title": "CJC Open",
"key": "10.1016/S2213-2600(22)00299-5_bib16",
"volume": "4",
"year": "2022"
},
{
"article-title": "Colchicine and the combination of rivaroxaban and aspirin in patients hospitalised with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial",
"author": "Eikelboom",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(22)00299-5_bib17",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(21)00435-5",
"article-title": "Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Group",
"doi-asserted-by": "crossref",
"first-page": "1419",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(22)00299-5_bib18",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.41328",
"article-title": "Effect of colchicine vs usual care alone on intubation and 28-day mortality in patients hospitalized with COVID-19: a randomized clinical trial",
"author": "Diaz",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/S2213-2600(22)00299-5_bib19",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"article-title": "Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: The GRECCO-19 Randomized Clinical Trial",
"author": "Deftereos",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/S2213-2600(22)00299-5_bib20",
"volume": "3",
"year": "2020"
},
{
"article-title": "Randomized controlled trial of colchicine add on to the standard therapy in moderate and severe corona virus Disease-19 infection",
"author": "Gorial",
"journal-title": "Ann Med Surg (Lond)",
"key": "10.1016/S2213-2600(22)00299-5_bib21",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.1016/S2352-3026(21)00105-8",
"article-title": "COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year",
"author": "Leentjens",
"doi-asserted-by": "crossref",
"first-page": "e524",
"journal-title": "Lancet Haematol",
"key": "10.1016/S2213-2600(22)00299-5_bib22",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1177/1740774509105223",
"article-title": "Evaluating the benefit of event adjudication of cardiovascular outcomes in large simple RCTs",
"author": "Pogue",
"doi-asserted-by": "crossref",
"first-page": "239",
"journal-title": "Clin Trials",
"key": "10.1016/S2213-2600(22)00299-5_bib23",
"volume": "6",
"year": "2009"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2213260022002995"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "Colchicine and aspirin in community patients with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}
eikelboom2