High-Dose Inhaled Nitric Oxide in Acute Hypoxemic Respiratory Failure Due to COVID-19: A Multicenter Phase II Trial
et al., American Journal of Respiratory and Critical Care Medicine, doi:10.1164/rccm.202304-0637OC, NCT04306393, Dec 2023
43rd treatment shown to reduce risk in
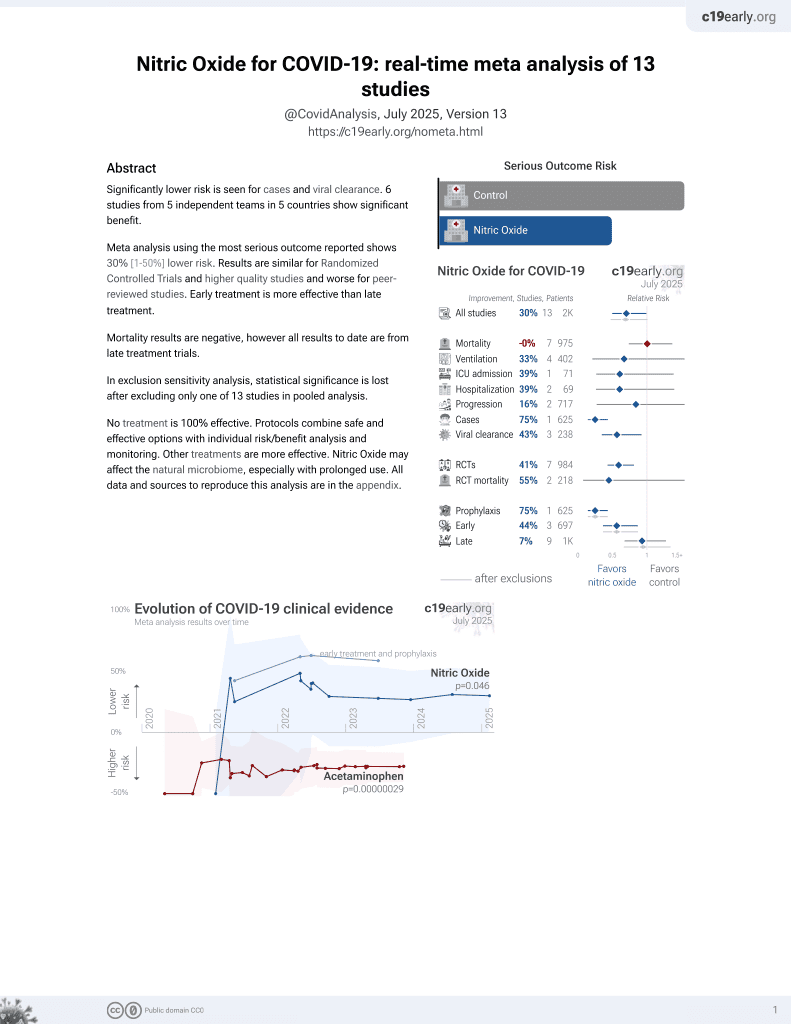
June 2022, now with p = 0.012 from 12 studies, recognized in 10 countries.
Lower risk for cases and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 193 mechanically ventilated COVID-19 patients showing improved oxygenation at 48 hours but no difference in mortality with high-dose (80ppm) inhaled nitric oxide (NO) for 48 hours. The NO group had a higher proportion attaining PaO2/FiO2 > 300 mmHg and reduced rates of neurologic symptoms at 90 days. NO was associated with faster viral clearance. No serious adverse events were reported with NO.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
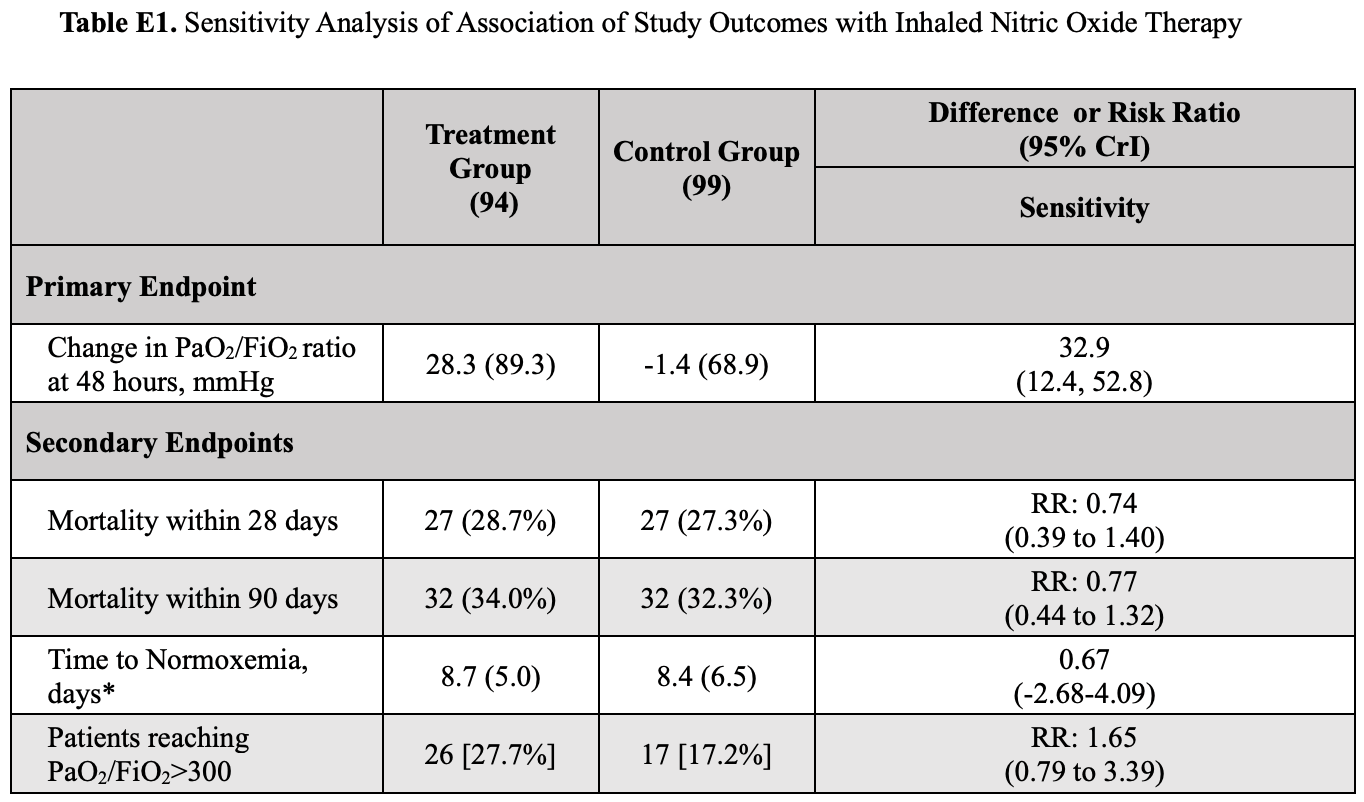
risk of death, 23.0% lower, RR 0.77, p = 0.36, treatment 94, control 99, including additional covariates with SMD > 0.20, day 90, Table E1.
|
|
risk of death, 26.0% lower, RR 0.74, p = 0.36, treatment 94, control 99, including additional covariates with SMD > 0.20, day 28, Table E1.
|
|
risk of death, 13.0% lower, RR 0.87, p = 0.60, treatment 94, control 99, day 90.
|
|
risk of death, 15.0% lower, RR 0.85, p = 0.56, treatment 94, control 99, day 28.
|
|
VV-ECMO, 30.0% lower, RR 0.70, p = 0.67, treatment 94, control 99.
|
|
neurological symptoms, 83.0% lower, RR 0.17, p = 0.01, treatment 94, control 99, day 90.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Di Fenza et al., 15 Dec 2023, Single Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 54 authors, study period 22 March, 2020 - 21 May, 2021, trial NCT04306393 (history).
Contact: dgern@thoracic.org, lberra@mgh.harvard.edu.
High-Dose Inhaled Nitric Oxide in Acute Hypoxemic Respiratory Failure Due to COVID-19: A Multicenter Phase II Trial
American Journal of Respiratory and Critical Care Medicine, doi:10.1164/rccm.202304-0637oc
Rationale: The effects of high-dose inhaled nitric oxide on hypoxemia in coronavirus disease (COVID-19) acute respiratory failure are unknown. Objectives: The primary outcome was the change in arterial oxygenation (Pa O 2 /FI O 2 ) at 48 hours. The secondary outcomes included: time to reach a Pa O 2 /FI O 2 .300mmHg for at least 24 hours, the proportion of participants with a Pa O 2 /FI O 2 .300mmHg at 28 days, and survival at 28 and at 90 days. Methods: Mechanically ventilated adults with COVID-19 pneumonia were enrolled in a phase II, multicenter, single-blind, randomized controlled parallel-arm trial. Participants in the intervention arm received inhaled nitric oxide at 80 ppm for 48 hours, compared with the control group receiving usual care (without placebo).
Measurements and Main Results: A total of 193 participants were included in the modified intention-to-treat analysis. The mean change in Pa O 2 /FI O 2 ratio at 48 hours was 28.3mmHg in the intervention group and 21.4mmHg in the control group (mean difference, 39.1mmHg; 95% credible interval [CrI], 18.1 to 60.3). The mean time to reach a Pa O 2 /FI O 2 .300mmHg in the interventional group was 8.7 days, compared with 8.4 days for the control group (mean difference, 0.44; 95% CrI, 23.63 to 4.53). At 28 days, the proportion of participants attaining a Pa O 2 /FI O 2 .300mmHg was 27.7% in the inhaled nitric oxide group and 17.2% in the control subjects (risk ratio, 2.03; 95% CrI, 1.11 to 3.86). Duration of ventilation and mortality at 28 and 90 days did not differ. No serious adverse events were reported. Conclusions: The use of high-dose inhaled nitric oxide resulted in an improvement of Pa O 2 /FI O 2 at 48 hours compared with usual care in adults with acute hypoxemic respiratory failure due to COVID-19.
Author disclosures are available with the text of this article at www.atsjournals.org.
Acknowledgment: The authors thank the participants, site staff, site investigators, and the entire Nitric Oxide Study Team. This work is dedicated to the recently deceased Prof. Warren M.
References
Adhikari, Dellinger, Lundin, Payen, Vallet et al., Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis, Crit Care Med
Akaberi, Krambrich, Ling, Luni, Hedenstierna, Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro, Redox Biol
Akerstr€ Om, Gunalan, Keng, Tan, Mirazimi, Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected, Virology
Andez-Castañeda, Lu, Geraghty, Song, Lee et al., Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation, Cell
Bartley, Gardner, Spina, Hurley, Campeau et al., High-dose inhaled nitric oxide as adjunct therapy in cystic fibrosis targeting Burkholderia multivorans, Case Rep Pediatr
Beckman, Bonillas, Diniz, Ott, Roh et al., SARS-CoV-2 infects neurons and induces neuroinflammation in a nonhuman primate model of COVID-19, Cell Rep
Brower, Matthay, Morris, Schoenfeld, Thompson et al., Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome, N Engl J Med
Chen, Liu, Gao, Sun, Chao et al., Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing, Clin Infect Dis
Dellinger, Zimmerman, Taylor, Straube, Hauser et al., Inhaled Nitric Oxide in ARDS Study Group. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial, Crit Care Med
Deppisch, Herrmann, Graepler-Mainka, Wirtz, Heyder et al., Gaseous nitric oxide to treat antibiotic resistant bacterial and fungal lung infections in patients with cystic fibrosis: a phase I clinical study, Infection
Fajnzylber, Regan, Coxen, Corry, Wong et al., Massachusetts Consortium for Pathogen Readiness. SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat Commun
Fakhr, Fenza, Gianni, Wiegand, Miyazaki et al., Nitric Oxide Study Investigators. Inhaled high dose nitric oxide is a safe and effective respiratory treatment in spontaneous breathing hospitalized patients with COVID-19 pneumonia, Nitric Oxide
Fang, Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity, J Clin Invest
Food, Administration, Drug approval package: INOmax (nitric oxide) NDA# 20-845
Frostell, Fratacci, Wain, Jones, Zapol, Inhaled nitric oxide: a selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction, Circulation
Gattinoni, Tognoni, Pesenti, Taccone, Mascheroni et al., Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure, N Engl J Med
Gerlach, Keh, Semmerow, Busch, Lewandowski et al., Dose-response characteristics during long-term inhalation of nitric oxide in patients with severe acute respiratory distress syndrome: a prospective, randomized, controlled study, Am J Respir Crit Care Med
Gerlach, Rossaint, Pappert, Falke, Time-course and doseresponse of nitric oxide inhalation for systemic oxygenation and pulmonary hypertension in patients with adult respiratory distress syndrome, Eur J Clin Invest
Gkaliagkousi, Ritter, Ferro, Platelet-derived nitric oxide signaling and regulation, Circ Res
Goldbart, Lavie, Lubetzky, Pillar, Landau et al., Inhaled nitric oxide for the treatment of acute bronchiolitis: a multicenter randomized controlled clinical trial to evaluate dose response, Ann Am Thorac Soc
Graham, Clark, Orban, Lim, Szymanski et al., Persistent neurologic symptoms and cognitive dysfunction in nonhospitalized Covid-19 "long haulers, Ann Clin Transl Neurol
Gu Erin, Reignier, Richard, Beuret, Gacouin et al., PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome, N Engl J Med
Guerin, Gaillard, Lemasson, Ayzac, Girard et al., Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial, JAMA
Ichinose, Roberts, Jr, Zapol, Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential, Circulation
Iotti, Olivei, Palo, Galbusera, Veronesi et al., Acute effects of inhaled nitric oxide in adult respiratory distress syndrome, Eur Respir J
Jean, Maître, Tankovic, Meignan, Adnot et al., Beneficial effects of nitric oxide inhalation on pulmonary bacterial clearance, Crit Care Med
Keyaerts, Vijgen, Chen, Maes, Hedenstierna et al., Inhibition of SARS-coronavirus infection in vitro by S-nitroso-Nacetylpenicillamine, a nitric oxide donor compound, Int J Infect Dis
Legrand, Bell, Forni, Joannidis, Koyner et al., Pathophysiology of COVID-19-associated acute kidney injury, Nat Rev Nephrol
Li, Schneider, Mehta, Sade-Feldman, Kays et al., SARS-CoV-2 viremia is associated with distinct proteomic pathways and predicts COVID-19 outcomes, J Clin Invest
Lundin, Mang, Smithies, Stenqvist, Frostell, The European Study Group of Inhaled Nitric Oxide. Inhalation of nitric oxide in acute lung injury: results of a European multicentre study, Intensive Care Med
Mcmullin, Chittock, Roscoe, Garcha, Wang et al., The antimicrobial effect of nitric oxide on the bacteria that cause nosocomial pneumonia in mechanically ventilated patients in the intensive care unit, Respir Care
Michael, Barton, Saffle, Mone, Markewitz et al., Inhaled nitric oxide versus conventional therapy: effect on oxygenation in ARDS, Am J Respir Crit Care Med
Mikkelsen, Christie, Lanken, Biester, Thompson et al., The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury, Am J Respir Crit Care Med
Miller, Hergott, Rohan, Arsenault-Mehta, D€ Oring et al., Inhaled nitric oxide decreases the bacterial load in a rat model of Pseudomonas aeruginosa pneumonia, J Cyst Fibros
Miller, Mcmullin, Ghaffari, Stenzler, Pick et al., Gaseous nitric oxide bactericidal activity retained during intermittent high-dose short duration exposure, Nitric Oxide
Peek, Mugford, Tiruvoipati, Wilson, Allen et al., CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial, Lancet
Peep, None, cm H 2 O
Regev-Shoshani, Vimalanathan, Mcmullin, Road, Av-Gay et al., Gaseous nitric oxide reduces influenza infectivity in vitro, Nitric Oxide
Roberts, Polaner, Lang, Zapol, Inhaled nitric oxide in persistent pulmonary hypertension of the newborn, Lancet
Rossaint, Falke, Slama, Pison, Zapol, Inhaled nitric oxide for the adult respiratory distress syndrome, N Engl J Med
Sinha, Calfee, Beitler, Soni, Ho et al., Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome, Am J Respir Crit Care Med
Talmor, Sarge, Malhotra, Donnell, Ritz et al., Mechanical ventilation guided by esophageal pressure in acute lung injury, N Engl J Med
Tandon, Wu, Moore, Winchester, Tu et al., Study Group. SARS-CoV-2 accelerated clearance using a novel nitric oxide nasal spray (NONS) treatment: a randomized trial, Lancet Reg Health Southeast Asia
Taylor, Zimmerman, Dellinger, Straube, Criner et al., Inhaled Nitric Oxide in ARDS Study Group. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial, JAMA
Troncy, Collet, Shapiro, Guimond, Blair et al., Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study, Am J Respir Crit Care Med
Valsecchi, Winterton, Fakhr, Collier, Nozari et al., DELiverly oF iNO (DELFiNO) Network Collaborators. High-dose inhaled nitric oxide for the treatment of spontaneously breathing pregnant patients with severe coronavirus disease 2019 (COVID-19) pneumonia, Obstet Gynecol
Villalba, Hilburn, Garlin, Elliott, Li et al., Vasculopathy and increased vascular congestion in fatal COVID-19 and acute respiratory distress syndrome, Am J Respir Crit Care Med
Wang, Cong, Miao, Yang, Zhang, Inhaled nitric oxide and acute kidney injury risk: a meta-analysis of randomized controlled trials, Ren Fail
Wiegand, Traeger, Nguyen, Rouillard, Fischbach et al., Antimicrobial effects of nitric oxide in murine models of Klebsiella pneumonia, Redox Biol
Xu, Xie, Al-Aly, Long-term neurologic outcomes of COVID-19, Nat Med
Zapol, Snider, Hill, Fallat, Bartlett et al., Extracorporeal membrane oxygenation in severe acute respiratory failure: a randomized prospective study, JAMA
DOI record:
{
"DOI": "10.1164/rccm.202304-0637oc",
"ISSN": [
"1073-449X",
"1535-4970"
],
"URL": "http://dx.doi.org/10.1164/rccm.202304-0637OC",
"alternative-id": [
"10.1164/rccm.202304-0637OC"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-04-03"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2023-09-28"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2023-12-15"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5675-7139",
"affiliation": [
{
"name": "Department of Anesthesia, Critical Care, and Pain Medicine,"
},
{
"name": "Harvard Medical School, Boston, Massachusetts;"
}
],
"authenticated-orcid": false,
"family": "Di Fenza",
"given": "Raffaele",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Division of Cardiovascular Disease, University of Alabama at Birmingham, Birmingham, Alabama;"
}
],
"family": "Shetty",
"given": "Naman S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0838-3654",
"affiliation": [
{
"name": "Department of Anesthesia, Critical Care, and Pain Medicine,"
},
{
"name": "Harvard Medical School, Boston, Massachusetts;"
}
],
"authenticated-orcid": false,
"family": "Gianni",
"given": "Stefano",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Cardiovascular Disease, University of Alabama at Birmingham, Birmingham, Alabama;"
}
],
"family": "Parcha",
"given": "Vibhu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Anesthesia, Critical Care, and Pain Medicine,"
},
{
"name": "Harvard Medical School, Boston, Massachusetts;"
}
],
"family": "Giammatteo",
"given": "Valentina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Anesthesia, Critical Care, and Pain Medicine,"
},
{
"name": "Harvard Medical School, Boston, Massachusetts;"
}
],
"family": "Safaee Fakhr",
"given": "Bijan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Sciences and"
},
{
"name": "Department of Anesthesia and Intensive Care and"
}
],
"family": "Tornberg",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Sciences and"
},
{
"name": "Department of Clinical Science and Education, Sodersxjukhuset, Karolinska Institutet, Stockholm, Sweden;"
}
],
"family": "Wall",
"given": "Olof",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Sciences and"
},
{
"name": "Department of Anesthesia and Intensive Care and"
}
],
"family": "Harbut",
"given": "Piotr",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9501-8606",
"affiliation": [
{
"name": "Pulmonary and Critical Care Medicine, Department of Medicine,"
},
{
"name": "Harvard Medical School, Boston, Massachusetts;"
}
],
"authenticated-orcid": false,
"family": "Lai",
"given": "Peggy S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard Medical School, Boston, Massachusetts;"
},
{
"name": "Division of Infectious Diseases, Brigham and Women’s Hospital, Boston, Massachusetts;"
}
],
"family": "Li",
"given": "Jonathan Z.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sean M. Healey and AMG Center for ALS,"
},
{
"name": "Neurological Clinical Research Institute,"
},
{
"name": "Harvard Medical School, Boston, Massachusetts;"
}
],
"family": "Paganoni",
"given": "Sabrina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Anesthesia, Critical Care, and Pain Medicine,"
},
{
"name": "Harvard Medical School, Boston, Massachusetts;"
}
],
"family": "Cenci",
"given": "Stefano",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Anesthesia, Critical Care, and Pain Medicine,"
},
{
"name": "Anesthesia Research Center,"
},
{
"name": "Harvard Medical School, Boston, Massachusetts;"
}
],
"family": "Mueller",
"given": "Ariel L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Anesthesia, Critical Care, and Pain Medicine,"
},
{
"name": "Anesthesia Research Center,"
},
{
"name": "Harvard Medical School, Boston, Massachusetts;"
}
],
"family": "Houle",
"given": "Timothy T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Anesthesia, Critical Care, and Pain Medicine,"
},
{
"name": "Harvard Medical School, Boston, Massachusetts;"
}
],
"family": "Akeju",
"given": "Oluwaseun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Anesthesia, Critical Care, and Pain Medicine,"
},
{
"name": "Harvard Medical School, Boston, Massachusetts;"
}
],
"family": "Bittner",
"given": "Edward A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard Medical School, Boston, Massachusetts;"
},
{
"name": "Department of Anesthesia, Critical Care, and Pain Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts; and"
}
],
"family": "Bose",
"given": "Somnath",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Critical Care Medicine, Department of Medicine, Louisiana State University Health Shreveport, Shreveport, Louisiana"
}
],
"family": "Scott",
"given": "Louie K.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7513-7023",
"affiliation": [
{
"name": "Division of Pediatric Critical Care Medicine, Department of Pediatrics,"
},
{
"name": "Harvard Medical School, Boston, Massachusetts;"
}
],
"authenticated-orcid": false,
"family": "Carroll",
"given": "Ryan W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Anesthesia, Critical Care, and Pain Medicine,"
},
{
"name": "Anesthesia Critical Care Center for Research, and"
},
{
"name": "Harvard Medical School, Boston, Massachusetts;"
}
],
"family": "Ichinose",
"given": "Fumito",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Danderyd Hospital, Stockholm, Sweden;"
}
],
"family": "Hedenstierna",
"given": "Magnus",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Cardiovascular Disease, University of Alabama at Birmingham, Birmingham, Alabama;"
}
],
"family": "Arora",
"given": "Pankaj",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2702-2093",
"affiliation": [
{
"name": "Department of Anesthesia, Critical Care, and Pain Medicine,"
},
{
"name": "Anesthesia Critical Care Center for Research, and"
},
{
"name": "Respiratory Care Services, Massachusetts General Hospital, Boston, Massachusetts;"
},
{
"name": "Harvard Medical School, Boston, Massachusetts;"
}
],
"authenticated-orcid": false,
"family": "Berra",
"given": "Lorenzo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morais",
"given": "Caio C. Araujo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gibson",
"given": "Lauren E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ikeda",
"given": "Takamitsu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marutani",
"given": "Eizo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miyazaki",
"given": "Yusuke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fischbach",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Traeger",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Capriles",
"given": "Martin I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Delgado",
"given": "Eduardo Diaz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Larson",
"given": "Grant M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santiago",
"given": "Roberta Ribeiro De Santis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vita",
"given": "Carolyn La",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yu",
"given": "Binglan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cereda",
"given": "Maurizio F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Greene",
"given": "Nattaly",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Restrepo",
"given": "Paula",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flynn",
"given": "James P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Regan",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pinciroli",
"given": "Riccardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Caskey",
"given": "Elizabeth I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hutchinson",
"given": "Kimberley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harris",
"given": "N. Stuart",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodriguez-Lopez",
"given": "Josanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chang",
"given": "Marvin G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wideaus",
"given": "Jacob",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Widaeus",
"given": "Matilda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shahgaldi",
"given": "Kambiz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hagman",
"given": "Karl",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arora",
"given": "Garima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johnson",
"given": "Robert",
"sequence": "additional"
}
],
"container-title": "American Journal of Respiratory and Critical Care Medicine",
"container-title-short": "Am J Respir Crit Care Med",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.atsjournals.org"
]
},
"created": {
"date-parts": [
[
2023,
9,
29
]
],
"date-time": "2023-09-29T18:01:32Z",
"timestamp": 1696010492000
},
"deposited": {
"date-parts": [
[
2023,
12,
15
]
],
"date-time": "2023-12-15T13:06:17Z",
"timestamp": 1702645577000
},
"funder": [
{
"DOI": "10.13039/100005294",
"doi-asserted-by": "publisher",
"name": "Massachusetts General Hospital"
},
{
"DOI": "10.13039/100005294",
"doi-asserted-by": "publisher",
"name": "Mallinckrodt Pharmaceuticals"
},
{
"DOI": "10.13039/100005294",
"award": [
"UM1AI069412"
],
"doi-asserted-by": "publisher",
"name": "Foundation for the NIH"
},
{
"DOI": "10.13039/100005294",
"doi-asserted-by": "publisher",
"name": "University of Alabama at Birmingham"
},
{
"DOI": "10.13039/100005294",
"doi-asserted-by": "publisher",
"name": "Beth Israel Deaconess Medical Center"
},
{
"DOI": "10.13039/100005294",
"doi-asserted-by": "publisher",
"name": "Louisiana State University Shreveport"
},
{
"name": "Danderyd Hospital"
},
{
"DOI": "10.13039/100027152",
"doi-asserted-by": "crossref",
"name": "Linde plc"
}
],
"indexed": {
"date-parts": [
[
2023,
12,
16
]
],
"date-time": "2023-12-16T00:39:02Z",
"timestamp": 1702687142854
},
"is-referenced-by-count": 2,
"issue": "12",
"issued": {
"date-parts": [
[
2023,
12,
15
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2023,
12,
15
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.atsjournals.org/doi/pdf/10.1164/rccm.202304-0637OC",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "19",
"original-title": [],
"page": "1293-1304",
"prefix": "10.1164",
"published": {
"date-parts": [
[
2023,
12,
15
]
]
},
"published-print": {
"date-parts": [
[
2023,
12,
15
]
]
},
"publisher": "American Thoracic Society",
"reference": [
{
"DOI": "10.1161/01.CIR.83.6.2038",
"doi-asserted-by": "publisher",
"key": "bib2"
},
{
"DOI": "10.1016/0140-6736(92)92686-A",
"doi-asserted-by": "publisher",
"key": "bib3"
},
{
"DOI": "10.1056/NEJM199302113280605",
"doi-asserted-by": "publisher",
"key": "bib6"
},
{
"DOI": "10.1001/jama.291.13.1603",
"doi-asserted-by": "publisher",
"key": "bib7"
},
{
"DOI": "10.1164/rccm.2108121",
"doi-asserted-by": "publisher",
"key": "bib8"
},
{
"DOI": "10.1111/j.1365-2362.1993.tb00797.x",
"doi-asserted-by": "publisher",
"key": "bib9"
},
{
"DOI": "10.1097/00003246-199801000-00011",
"doi-asserted-by": "publisher",
"key": "bib10"
},
{
"DOI": "10.1164/ajrccm.157.5.9707090",
"doi-asserted-by": "publisher",
"key": "bib11"
},
{
"DOI": "10.1007/s001340050982",
"doi-asserted-by": "publisher",
"key": "bib12"
},
{
"DOI": "10.1164/ajrccm.157.5.96-10089",
"doi-asserted-by": "publisher",
"key": "bib13"
},
{
"DOI": "10.1016/j.redox.2020.101826",
"doi-asserted-by": "publisher",
"key": "bib14"
},
{
"DOI": "10.1172/JCI119473",
"doi-asserted-by": "publisher",
"key": "bib15"
},
{
"DOI": "10.1016/j.redox.2020.101734",
"doi-asserted-by": "publisher",
"key": "bib16"
},
{
"DOI": "10.1016/j.ijid.2004.04.012",
"doi-asserted-by": "publisher",
"key": "bib17"
},
{
"DOI": "10.1097/CCM.0b013e3182a27909",
"doi-asserted-by": "publisher",
"key": "bib18"
},
{
"DOI": "10.1097/AOG.0000000000004847",
"doi-asserted-by": "publisher",
"key": "bib19"
},
{
"DOI": "10.1513/AnnalsATS.202103-348OC",
"doi-asserted-by": "publisher",
"key": "bib20"
},
{
"author": "Bartley BL",
"first-page": "1536714",
"journal-title": "Case Rep Pediatr",
"key": "bib21",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1086/425357",
"doi-asserted-by": "publisher",
"key": "bib22"
},
{
"DOI": "10.1016/j.niox.2013.03.007",
"doi-asserted-by": "publisher",
"key": "bib23"
},
{
"DOI": "10.1007/s15010-016-0879-x",
"doi-asserted-by": "publisher",
"key": "bib24"
},
{
"DOI": "10.1016/j.jcf.2013.01.008",
"doi-asserted-by": "publisher",
"key": "bib25"
},
{
"DOI": "10.1097/00003246-200202000-00029",
"doi-asserted-by": "publisher",
"key": "bib26"
},
{
"DOI": "10.1016/j.niox.2008.08.002",
"doi-asserted-by": "publisher",
"key": "bib27"
},
{
"author": "McMullin BB",
"first-page": "1451",
"journal-title": "Respir Care",
"key": "bib28",
"volume": "50",
"year": "2005"
},
{
"DOI": "10.1056/NEJMoa0708638",
"doi-asserted-by": "publisher",
"key": "bib29"
},
{
"DOI": "10.1080/0886022X.2021.1873805",
"doi-asserted-by": "publisher",
"key": "bib30"
},
{
"DOI": "10.1038/s41581-021-00452-0",
"doi-asserted-by": "publisher",
"key": "bib31"
},
{
"DOI": "10.1056/NEJM200005043421801",
"doi-asserted-by": "publisher",
"key": "bib32"
},
{
"DOI": "10.1056/NEJMoa1214103",
"doi-asserted-by": "publisher",
"key": "bib33"
},
{
"DOI": "10.1001/jama.292.19.2379",
"doi-asserted-by": "publisher",
"key": "bib34"
},
{
"DOI": "10.1056/NEJMoa010043",
"doi-asserted-by": "publisher",
"key": "bib35"
},
{
"DOI": "10.1001/jama.1979.03300200023016",
"doi-asserted-by": "publisher",
"key": "bib36"
},
{
"DOI": "10.1016/S0140-6736(09)61069-2",
"doi-asserted-by": "publisher",
"key": "bib37"
},
{
"DOI": "10.1164/rccm.202109-2150OC",
"doi-asserted-by": "publisher",
"key": "bib38"
},
{
"DOI": "10.1164/rccm.201804-0692OC",
"doi-asserted-by": "publisher",
"key": "bib39"
},
{
"DOI": "10.1161/CIRCRESAHA.107.158410",
"doi-asserted-by": "publisher",
"key": "bib40"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"doi-asserted-by": "publisher",
"key": "bib41"
},
{
"DOI": "10.1172/JCI148635",
"doi-asserted-by": "publisher",
"key": "bib42"
},
{
"DOI": "10.1016/j.lansea.2022.100036",
"doi-asserted-by": "publisher",
"key": "bib43"
},
{
"DOI": "10.1016/j.virol.2009.09.007",
"doi-asserted-by": "publisher",
"key": "bib44"
},
{
"DOI": "10.1016/j.niox.2021.08.003",
"doi-asserted-by": "publisher",
"key": "bib45"
},
{
"DOI": "10.1016/j.celrep.2022.111573",
"doi-asserted-by": "publisher",
"key": "bib46"
},
{
"DOI": "10.1016/j.cell.2022.06.008",
"doi-asserted-by": "publisher",
"key": "bib47"
},
{
"DOI": "10.1038/s41591-022-02001-z",
"doi-asserted-by": "publisher",
"key": "bib48"
},
{
"DOI": "10.1161/01.CIR.0000134595.80170.62",
"doi-asserted-by": "publisher",
"key": "bib49"
},
{
"DOI": "10.1002/acn3.51350",
"doi-asserted-by": "publisher",
"key": "bib50"
},
{
"DOI": "10.1164/rccm.201111-2025OC",
"doi-asserted-by": "publisher",
"key": "bib51"
},
{
"DOI": "10.1183/09031936.98.12051164",
"doi-asserted-by": "publisher",
"key": "bib52"
}
],
"reference-count": 49,
"references-count": 49,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.atsjournals.org/doi/10.1164/rccm.202304-0637OC"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Critical Care and Intensive Care Medicine",
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "High-Dose Inhaled Nitric Oxide in Acute Hypoxemic Respiratory Failure Due to COVID-19: A Multicenter Phase II Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1164/crossmarkpolicies",
"volume": "208"
}