Effect of Colchicine vs Usual Care Alone on Intubation and 28-Day Mortality in Patients Hospitalized With COVID-19
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2021.41328, NCT04328480, Dec 2021
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Very late stage RCT (O2 88%, 84% on oxygen) with 1,279 hospitalized patients in Argentina, showing lower mortality and lower combined mortality/ventilation, statistically significant only for the combined outcome and per-protocol analysis. NCT04328480 (history). COLCOVID.
This study is excluded in the after exclusion results of meta-analysis:
very late stage, oxygen saturation <90% at baseline; very late stage, >80% on oxygen/ventilation at baseline.
|
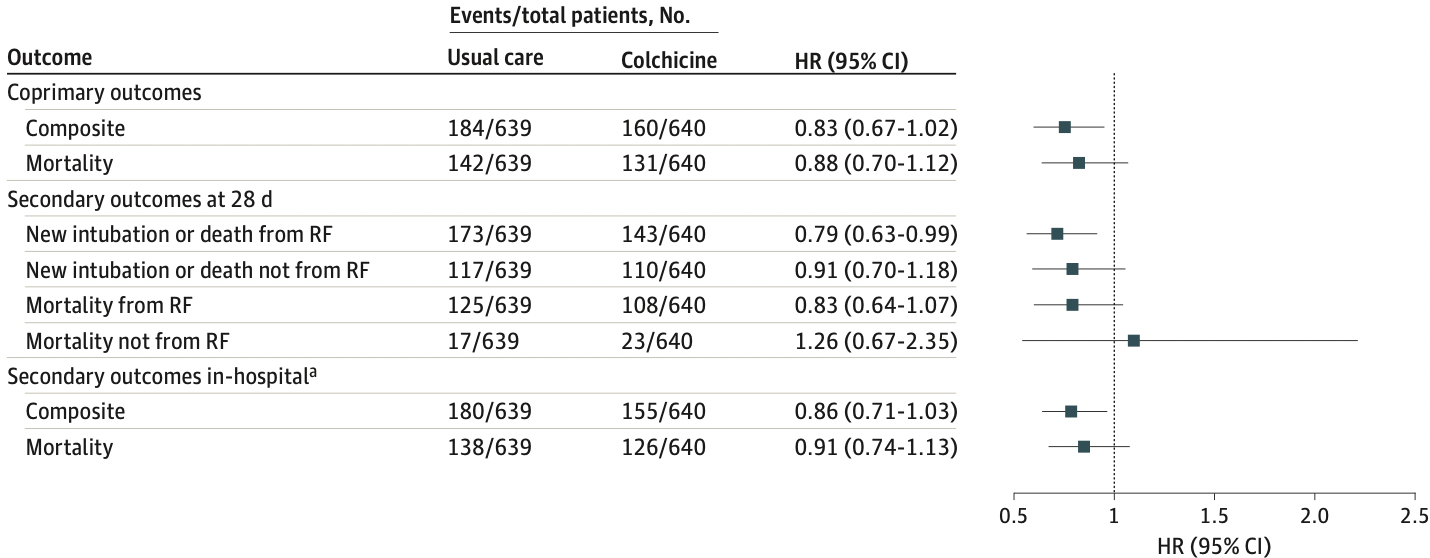
risk of death, 12.0% lower, HR 0.88, p = 0.30, treatment 131 of 640 (20.5%), control 142 of 639 (22.2%), NNT 57, adjusted per study, Cox proportional hazards, primary outcome.
|
|
risk of death/intubation, 17.0% lower, HR 0.83, p = 0.08, treatment 160 of 640 (25.0%), control 184 of 639 (28.8%), NNT 26, adjusted per study, Cox proportional hazards, primary outcome.
|
|
risk of death/intubation, 52.0% lower, HR 0.48, p = 0.60, treatment 6 of 93 (6.5%), control 13 of 102 (12.7%), NNT 16, adjusted per study, subset not on supplemental oxygen, Cox proportional hazards.
|
|
risk of death, 17.0% lower, HR 0.83, p = 0.30, treatment 98 of 515 (19.0%), control 140 of 634 (22.1%), NNT 33, adjusted per study, PP, Cox proportional hazards.
|
|
risk of death/intubation, 25.0% lower, HR 0.75, p = 0.02, treatment 117 of 515 (22.7%), control 181 of 634 (28.5%), NNT 17, adjusted per study, PP, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Diaz et al., 29 Dec 2021, Randomized Controlled Trial, Argentina, peer-reviewed, 101 authors, study period 17 April, 2020 - 28 March, 2021, dosage 2mg day 1, 1mg days 2-14, trial NCT04328480 (history).
Effect of Colchicine vs Usual Care Alone on Intubation and 28-Day Mortality in Patients Hospitalized With COVID-19
JAMA Network Open, doi:10.1001/jamanetworkopen.2021.41328
IMPORTANCE Hospitalized patients with COVID-19 pneumonia have high rates of morbidity and mortality. OBJECTIVE To assess the efficacy of colchicine in hospitalized patients with COVID-19 pneumonia. DESIGN, SETTING, AND PARTICIPANTS The Estudios Clínicos Latino América (ECLA) Population Health Research Institute (PHRI) COLCOVID trial was a multicenter, open-label, randomized clinical trial performed from April 17, 2020, to March 28, 2021, in adults with confirmed or suspected SARS-CoV-2 infection followed for up to 28 days. Participants received colchicine vs usual care if they were hospitalized with COVID-19 symptoms and had severe acute respiratory syndrome or oxygen desaturation. The main exclusion criteria were clear indications or contraindications for colchicine, chronic kidney disease, and negative results on a reverse transcription-polymerase chain reaction test for SARS-CoV-2 before randomization. Data were analyzed from June 20 to July 25, 2021. INTERVENTIONS Patients were assigned in a 1:1 ratio to usual care or usual care plus colchicine. Colchicine was administered orally in a loading dose of 1.5 mg immediately after randomization, followed by 0.5 mg orally within 2 hours of the initial dose and 0.5 mg orally twice a day for 14 days or discharge, whichever occurred first.
MAIN OUTCOMES AND MEASURES The first coprimary outcome was the composite of a new requirement for mechanical ventilation or death evaluated at 28 days. The second coprimary outcome was death at 28 days. RESULTS A total of 1279 hospitalized patients (mean [SD] age, 61.8 [14.6] years; 449 [35.1%] women and 830 [64.9%] men) were randomized, including 639 patients in the usual care group and 640 patients in the colchicine group. Corticosteroids were used in 1171 patients (91.5%). The coprimary outcome of mechanical ventilation or 28-day death occurred in 160 patients (25.0%) in the colchicine group and 184 patients (28.8%) in the usual care group (hazard ratio [HR], 0.83; 95% CI, 0.67-1.02; P = .08). The second coprimary outcome, 28-day death, occurred in 131 patients (20.5%) in the colchicine group and 142 patients (22.2%) in the usual care group (HR, 0.88; 95% CI, 0.70-1.12). Diarrhea was the most frequent adverse effect of colchicine, reported in 68 patients (11.3%).
Conflict of Interest Disclosures: Dr Orlandini reported receiving grants from Population Health Research Institution during the conduct of the study. Dr Lamelas reported receiving personal fees from Boston Scientific, Medtronic, Edwards, and Meril outside the submitted work. Dr Miró reported receiving grants from Angelini, Contrafect, Gilead Sciences, MSD, Novartis, Pfizer, and ViiV Healthcare and personal fees from Gilead Sciences, Jansen, Lysovant, Medtronic, MSD, Novartis, and Pfizer outside the submitted work. Dr Eikelboom reported receiving grants and personal fees from Astra-Zeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Pfizer, Janssen, and Sanofi-Aventis and personal fees from Daiichi-Sankyo, Eli-Lilly, and Servier during the conduct of the study. Funding/Support: The Population Health Research Institute contributed fees to the investigators. Fundacion ECLA funded all other aspects of the trial. Role of the Funder/Sponsor: Fundacion ECLA was responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
Administrative, Orlandini, Castellana, Caccavo, Corral et al., None
Gaba, Bhatt, The COVID-19 pandemic: a catalyst to improve clinical trials, Nat Rev Cardiol, doi:10.1038/s41569-020-00439-7
Ghosn, Chaimani, Evrenoglou, Interleukin-6 blocking agents for treating COVID-19: a living systematic review, Cochrane Database Syst Rev, doi:10.1002/14651858.CD013881
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform, doi:10.1016/j.jbi.2008.08.010
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Medical, World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects, JAMA, doi:https://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2013.281053&utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamanetworkopen.2021.41328
Moore, June, Cytokine release syndrome in severe COVID-19, Science, doi:10.1126/science.abb8925
Nidorf, Fiolet, Mosterd, LoDoCo2 Trial Investigators. Colchicine in patients with chronic coronary disease, N Engl J Med, doi:10.1056/NEJMoa2021372
Orlandini, Castellana, Domínguez, Scarafia, Eikelboom, Obtained funding: Orlandini
Pan, Peto, Henao-Restrepo, WHO Solidarity Trial Consortium. Repurposed antiviral drugs for COVID-19-interim WHO solidarity trial results, N Engl J Med, doi:10.1056/NEJMoa2023184
Reyes, Hu, Teperman, Anti-inflammatory therapy for COVID-19 infection: the case for colchicine, Ann Rheum Dis, doi:10.1136/annrheumdis-2020-219174
Richardson, Hirsch, Narasimhan, the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area, JAMA, doi:https://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2020.6775&utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamanetworkopen.2021.41328
Tardif, Bouabdallaoui, Allier, Efficacy of colchicine in non-hospitalized patients with COVID-19. medRxiv, doi:10.1101/2021.01.26.21250494
Tardif, Kouz, Waters, Efficacy and safety of low-dose colchicine after myocardial infarction, N Engl J Med, doi:10.1056/NEJMoa1912388
Yusuf, Collins, Peto, Why do we need some large, simple randomized trials?, Stat Med, doi:10.1002/sim.4780030421
DOI record:
{
"DOI": "10.1001/jamanetworkopen.2021.41328",
"ISSN": [
"2574-3805"
],
"URL": "http://dx.doi.org/10.1001/jamanetworkopen.2021.41328",
"author": [
{
"affiliation": [
{
"name": "Estudios Clínicos Latino América, Rosario, Argentina"
},
{
"name": "Instituto Cardiovascular de Rosario, Rosario, Argentina"
}
],
"family": "Diaz",
"given": "Rafael",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Estudios Clínicos Latino América, Rosario, Argentina"
},
{
"name": "Instituto Cardiovascular de Rosario, Rosario, Argentina"
}
],
"family": "Orlandini",
"given": "Andrés",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Estudios Clínicos Latino América, Rosario, Argentina"
},
{
"name": "Universidad Nacional de Rosario, Rosario, Argentina"
}
],
"family": "Castellana",
"given": "Noelia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital de Coronel Suárez Raúl Alfredo Caccavo, Universidad Provincial del Sudoeste, Buenos Aires, Argentina"
}
],
"family": "Caccavo",
"given": "Alberto",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Departamento de Investigación, Facultad de Medicina, Universidad FASTA, Buenos Aires, Argentina"
}
],
"family": "Corral",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectología Clínica de Mayo, Mar del Plata, Argentina"
}
],
"family": "Corral",
"given": "Gonzalo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Estudios Clínicos Latino América, Rosario, Argentina"
},
{
"name": "Universidad Abierta Interamericana, Rosario, Argentina"
},
{
"name": "Unidad Coronaria de Sanatorio Delta de Rosario, Rosario, Argentina"
},
{
"name": "Comite de Epidemiologia y Prevención Cardiovasculr de la Federación Argentina de Cardiologia, Rosario, Argentina"
}
],
"family": "Chacón",
"given": "Carolina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Health Research Methods, Evidence, and Impact, Population Health Research Institute, Hamilton Health Sciences, McMaster University, Hamilton, Canada"
},
{
"name": "Instituto Cardiovascular de Buenos Aires, Buenos Aires, Argentina"
}
],
"family": "Lamelas",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Instituto Cardiovascular de Buenos Aires, Buenos Aires, Argentina"
}
],
"family": "Botto",
"given": "Fernando",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Estudios Clínicos Latino América, Rosario, Argentina"
},
{
"name": "Instituto Cardiovascular de Rosario, Rosario, Argentina"
}
],
"family": "Díaz",
"given": "María Luz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Estudios Clínicos Latino América, Rosario, Argentina"
},
{
"name": "Instituto Cardiovascular de Rosario, Rosario, Argentina"
},
{
"name": "Heart Failure and Heart Transplant Unit, Instituto Cardiovascular de Rosario, Rosario, Argentina"
}
],
"family": "Domínguez",
"given": "Juan Manuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Estudios Clínicos Latino América, Rosario, Argentina"
}
],
"family": "Pascual",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Estudios Clínicos Latino América, Rosario, Argentina"
}
],
"family": "Rovito",
"given": "Carla",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Estudios Clínicos Latino América, Rosario, Argentina"
}
],
"family": "Galatte",
"given": "Agustina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Estudios Clínicos Latino América, Rosario, Argentina"
},
{
"name": "Statistics Department, Universidad Nacional de Rosario, Rosario, Argentina"
}
],
"family": "Scarafia",
"given": "Franco",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fundación Huésped, Buenos Aires, Argentina"
}
],
"family": "Sued",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ministerio de Salud de Jujuy, Jujuy, Argentina"
}
],
"family": "Gutierrez",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Cardiology, Population Health Research Institute, Hamilton Health Sciences, McMaster University, Hamilton, Canada"
}
],
"family": "Jolly",
"given": "Sanjit S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Service, Hospital Clínic, Instituto de Investigaciones Biomédicas August Pi i Sunyer, University of Barcelona, Barcelona, Spain"
}
],
"family": "Miró",
"given": "José M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medicine, Population Health Research Institute, Hamilton Health Sciences, McMaster University, Hamilton, Canada"
}
],
"family": "Eikelboom",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Health Research Methods, Evidence, and Impact, Population Health Research Institute, Hamilton Health Sciences, McMaster University, Hamilton, Canada"
},
{
"name": "Departments of Pathology and Molecular Medicine, Population Health Research Institute, Hamilton Health Sciences, McMaster University, Hamilton, Canada"
}
],
"family": "Loeb",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Associazione Nazionale Medici Cardiologi Ospedalieri Research Center, Florence, Italy"
}
],
"family": "Maggioni",
"given": "Aldo Pietro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts"
}
],
"family": "Bhatt",
"given": "Deepak L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Cardiology, Population Health Research Institute, Hamilton Health Sciences, McMaster University, Hamilton, Canada"
}
],
"family": "Yusuf",
"given": "Salim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Lopez",
"given": "Lorena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Muntaner",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Bobato",
"given": "Antonela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Corral",
"given": "Gonzalo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Benavent",
"given": "Gustavo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Espinel",
"given": "Diego",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Del Valle Almagro",
"given": "Sandra M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Montenegro",
"given": "Eleonora E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Núñez",
"given": "Adrian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Pérez Valega",
"given": "Lisandro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Christin",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Guzzi",
"given": "Leda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Finelli",
"given": "Gabriela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Schiavi",
"given": "Lilina B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Ferro Queirel",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Moltrasio",
"given": "Luis M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Fermín",
"given": "Horacio A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Martínez",
"given": "Jorge V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Gutiérrez",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Cunto",
"given": "Eleonora",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Saúl",
"given": "Pablo A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Cabrera Maciel",
"given": "María del Pilar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Muntaner",
"given": "Javier",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Lerman",
"given": "Damián",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Truccolo",
"given": "Paula I.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Armano",
"given": "Adrián",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Jalife",
"given": "Esther V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Bertuzzi",
"given": "Romina M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Jean Charles",
"given": "María Inés",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Fernandez",
"given": "Pablo A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Caccavo",
"given": "Alberto",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Vittal",
"given": "Nicolás",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Lampone Tappata",
"given": "Lucia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Murizzi",
"given": "Diego M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Fernandez",
"given": "Brenda L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Montes de Oca",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Diaz Vega",
"given": "Guadalupe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Queti",
"given": "Felipe N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Calafell",
"given": "Luis A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Sequeira",
"given": "Mariano",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "León de la Fuente",
"given": "Ricardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Núñez Burgos",
"given": "Julio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Del Valle Armaraz",
"given": "Sonia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Flores",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Bellanting",
"given": "Mariana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Blazevich",
"given": "Narela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Finucci Curi",
"given": "Baltasar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Cabrini",
"given": "Romina P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Langone",
"given": "Martín E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Figueroa",
"given": "Álvaro E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Iglesias",
"given": "Maria T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Alvero",
"given": "Maria Carolina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Lemir",
"given": "Cesar G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Bonorino",
"given": "José",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Pereyra",
"given": "María Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Barral",
"given": "Ezequiel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Rasmussen",
"given": "Mariela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Daglio",
"given": "María F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Estofan",
"given": "Mariano D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Perea",
"given": "Francisco M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Duhalde",
"given": "Sebastián E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Motta",
"given": "María Fernanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Romero",
"given": "Ignacio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Isa Massa",
"given": "Guillermo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "García",
"given": "Celso F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "García Durán",
"given": "Rubén",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Cornejo Pucci",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Saavedra",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Bozikovich",
"given": "Carolina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Lovesio",
"given": "Luciano",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Fernandez Moutin",
"given": "María J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Forciniti",
"given": "Cristian C. G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Colombo",
"given": "Hugo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Sabas",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Pilón",
"given": "Leonardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ECLA PHRI COLCOVID Trial Investigators"
}
],
"family": "Steren",
"given": "Adriana P.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "ECLA PHRI COLCOVID Trial Investigators",
"sequence": "additional"
}
],
"container-title": [
"JAMA Network Open"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
29
]
],
"date-time": "2021-12-29T17:09:30Z",
"timestamp": 1640797770000
},
"deposited": {
"date-parts": [
[
2021,
12,
29
]
],
"date-time": "2021-12-29T17:09:37Z",
"timestamp": 1640797777000
},
"indexed": {
"date-parts": [
[
2022,
2,
3
]
],
"date-time": "2022-02-03T03:27:59Z",
"timestamp": 1643858879045
},
"is-referenced-by-count": 1,
"issn-type": [
{
"type": "electronic",
"value": "2574-3805"
}
],
"issue": "12",
"issued": {
"date-parts": [
[
2021,
12,
29
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2021,
12,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamanetworkopen/articlepdf/2787585/diaz_2021_oi_211156_1640104700.33987.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "e2141328",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2021,
12,
29
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
29
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area.",
"author": "Richardson",
"doi-asserted-by": "publisher",
"first-page": "2052",
"issue": "20",
"journal-title": "JAMA",
"key": "zoi211156r1",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with COVID-19.",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "zoi211156r2",
"volume": "384",
"year": "2021"
},
{
"article-title": "Interleukin-6 blocking agents for treating COVID-19: a living systematic review.",
"author": "Ghosn",
"journal-title": "Cochrane Database Syst Rev",
"key": "zoi211156r3",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2023184",
"article-title": "Repurposed antiviral drugs for COVID-19—interim WHO solidarity trial results.",
"author": "Pan",
"doi-asserted-by": "crossref",
"first-page": "497",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "zoi211156r4",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00149-5",
"article-title": "Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial.",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "605",
"issue": "10274",
"journal-title": "Lancet",
"key": "zoi211156r5",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1126/science.abb8925",
"article-title": "Cytokine release syndrome in severe COVID-19.",
"author": "Moore",
"doi-asserted-by": "publisher",
"first-page": "473",
"issue": "6490",
"journal-title": "Science",
"key": "zoi211156r6",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1136/annrheumdis-2020-219174",
"article-title": "Anti-inflammatory therapy for COVID-19 infection: the case for colchicine.",
"author": "Reyes",
"doi-asserted-by": "publisher",
"first-page": "550",
"issue": "5",
"journal-title": "Ann Rheum Dis",
"key": "zoi211156r8",
"volume": "80",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa1912388",
"article-title": "Efficacy and safety of low-dose colchicine after myocardial infarction.",
"author": "Tardif",
"doi-asserted-by": "publisher",
"first-page": "2497",
"issue": "26",
"journal-title": "N Engl J Med",
"key": "zoi211156r9",
"volume": "381",
"year": "2019"
},
{
"DOI": "10.1056/NEJMoa2021372",
"article-title": "Colchicine in patients with chronic coronary disease.",
"author": "Nidorf",
"doi-asserted-by": "publisher",
"first-page": "1838",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "zoi211156r10",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1002/(ISSN)1097-0258",
"article-title": "Why do we need some large, simple randomized trials?",
"author": "Yusuf",
"doi-asserted-by": "publisher",
"first-page": "409",
"issue": "4",
"journal-title": "Stat Med",
"key": "zoi211156r11",
"volume": "3",
"year": "1984"
},
{
"DOI": "10.1038/s41569-020-00439-7",
"article-title": "The COVID-19 pandemic: a catalyst to improve clinical trials.",
"author": "Gaba",
"doi-asserted-by": "publisher",
"first-page": "673",
"issue": "11",
"journal-title": "Nat Rev Cardiol",
"key": "zoi211156r12",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1001/jama.2013.281053",
"article-title": "World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects.",
"author": "World Medical Association",
"doi-asserted-by": "publisher",
"first-page": "2191",
"issue": "20",
"journal-title": "JAMA",
"key": "zoi211156r13",
"volume": "310",
"year": "2013"
},
{
"DOI": "10.1016/j.jbi.2008.08.010",
"article-title": "Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support.",
"author": "Harris",
"doi-asserted-by": "publisher",
"first-page": "377",
"issue": "2",
"journal-title": "J Biomed Inform",
"key": "zoi211156r14",
"volume": "42",
"year": "2009"
},
{
"article-title": "Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial.",
"author": "RECOVERY Collaborative Group",
"journal-title": "Lancet Resp Med",
"key": "zoi211156r15",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"article-title": "Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial.",
"author": "Deftereos",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "JAMA Netw Open",
"key": "zoi211156r17",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1136/rmdopen-2020-001455",
"article-title": "Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial.",
"author": "Lopes",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "RMD Open",
"key": "zoi211156r18",
"volume": "7",
"year": "2021"
},
{
"key": "zoi211156r7",
"unstructured": "University of Oxford. RECOVERY: Randomised Evaluation of COVID-19 Therapy. Accessed June 11, 2021. https://www.recoverytrial.net/"
},
{
"DOI": "10.1101/2021.01.26.21250494",
"doi-asserted-by": "crossref",
"key": "zoi211156r16",
"unstructured": "Tardif? JC, Bouabdallaoui? N, L’Allier? PL, ? Efficacy of colchicine in non-hospitalized patients with COVID-19.? medRxiv. Preprint posted online January 27, 2021. doi:10.1101/2021.01.26.21250494"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"score": 1,
"short-container-title": [
"JAMA Netw Open"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": [
"Effect of Colchicine vs Usual Care Alone on Intubation and 28-Day Mortality in Patients Hospitalized With COVID-19"
],
"type": "journal-article",
"volume": "4"
}
