To estimate the efficacy of inhaled corticosteroid in-patient of moderate COVID-19 pneumonia - randomized controlled study
et al., International Journal of Academic Medicine and Pharmacy, doi:10.47009/jamp.2023.5.5.101, REF/2021/09/046997, Sep 2023
Budesonide for COVID-19
27th treatment shown to reduce risk in
September 2021, now with p = 0.0000042 from 14 studies, recognized in 10 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
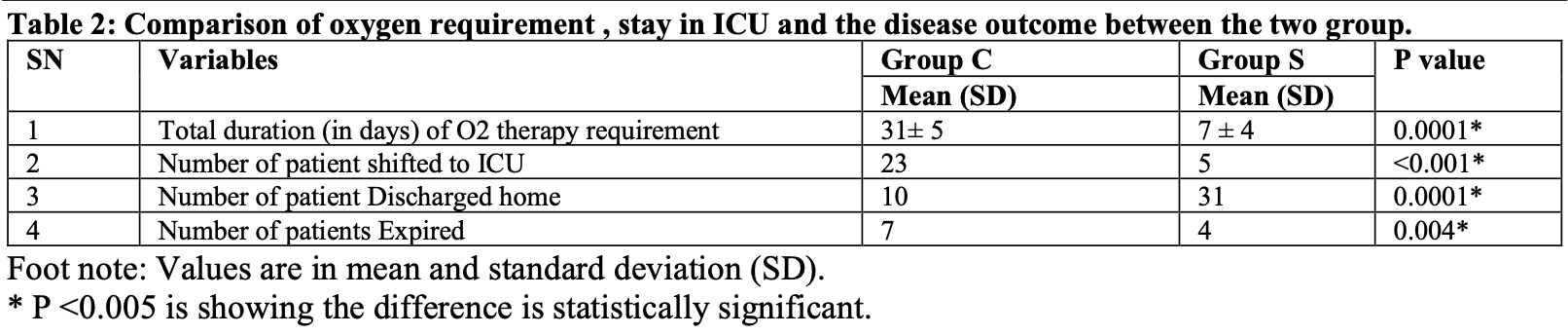
RCT inhaled budesonide with 80 moderate COVID-19 pneumonia patients. The budesonide group had significantly faster time to clinical improvement, fewer ICU admissions, shorter oxygen therapy duration, and lower mortality. Inhaled budesonide 400mcg twice daily for 14 days.
|
risk of death, 42.9% lower, RR 0.57, p = 0.52, treatment 4 of 40 (10.0%), control 7 of 40 (17.5%), NNT 13.
|
|
risk of ICU admission, 78.3% lower, RR 0.22, p < 0.001, treatment 5 of 40 (12.5%), control 23 of 40 (57.5%), NNT 2.2.
|
|
risk of no recovery, 70.0% lower, RR 0.30, p < 0.001, treatment 9 of 40 (22.5%), control 30 of 40 (75.0%), NNT 1.9.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Dhanger et al., 30 Sep 2023, Randomized Controlled Trial, India, peer-reviewed, 4 authors, study period January 2022 - March 2022, trial REF/2021/09/046997.
TO ESTIMATE THE EFFICACY OF INHALED CORTICOSTEROID IN-PATIENT OF MODERATE COVID -19 PNEUMONIA-RANDOMIZED CONTROLLED STUDY
doi:10.47009/jamp.2023.5.5.101
Background: Systemic corticosteroids have been found to reduce the mortality in patients with moderated and severe COVID-19 pneumonia in the first and second waves of the pandemic but had the unwanted effects which can be avoided when the corticosteroids are given via the inhalational route (ICS). Therefore in this study, we aimed to investigate whether Inhaled Corticosteroid MDI -Budesonide could reduce the morbidity in patients with moderate COVID-19 pneumonia. Our primary objective was to study the time to clinical improvement in days (Time Frame: Up to 14 days)defined as the resolution of all systemic and respiratory symptoms (SPO2 > 92% in room air, RR 14-16/min, for ≥2 consecutive days). Materials and Methods: After obtaining approval from Institute Research and Ethics committee and following CTRI registration, this randomized controlled study was carried out from January to March 2022 in a covid dedicated hospital. Eighty participants were enrolled and written informed consent was obtained. The study interventions began within 6-12 h of confirmation of diagnosis with RT PCR report and CT scan of thorax (CORAD Score 2-3) of moderate COVID 19. Participants were randomized by the computer-generated random number table and were divided in to two groups: S (Intervention) group and C (control) group. Both the groups received the Covid 19 care as per the hospital treatment protocol. In addition, participants of Group S received Inhaled Budesonide MDI (metered dose inhaler) 2 puff (400mcg) two times in a day for 14 days. All patients were hourly monitored and following parameters were recorded daily for 14 days: GCS, HR, NIBP, SPO2, HR, Temperature, mode of Oxygen therapy: (FM/ VM/ NRBM/ NIV/ IV) and FIO2. Study end point may be before 14 days incase of patient was shifted to ICU due to requirement of mechanical ventilation or incase of adverse outcome within 14 days. Result: The results of this study showed that budesonide nebulization significantly reduced morbidity as there were significant difference between both the group in the number of days requirement of oxygen in Group C 31± 5 days and Group S 7 ± 4 days, number of patient shifted to ICU in Group C 23 and Group S 5 and number of patients expired in Group C 13 and Group S only 4. Discussion is Budesonide is an inhaled glucocorticoid, which inhibits a variety of inflammatory cells, reduces the production of inflammatory mediators and consequently has a significant antiinflammatory effect. Compared to systemic glucocorticoids, budesonide inhalation has the following advantages; high concentration primarily in the lungs, high hepatic clearance, and high glucocorticoid receptor affinity. During nebulization, the nebulizer unit breaks the liquid into micro-particles, which are directly inhaled into the lower respiratory tract and rapidly absorbed by the pulmonary mucosa, thus increasing the local drug concentration. Conclusion: Inhaled budesonide in patient with moderate covid pneumonia..
References
Al-Tawfiq, Alhumaid, Alshukairi, COVID-19 and mucormycosis superinfection: the perfect storm, Infection
Berlinski, Year in Review: Aerosol Therapy, Respir Care
Brown, Dean, Defining severe pneumonia, Clin Chest Med
Chen, Zhou, Dong, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, Lancet
Cruciani, Pati, Masiello, Pupella, Angelis, Corticosteroids use for COVID-19: an overview of systematic reviews, Infez Med
Eedara, Alabsi, Encinas-Basurto, Inhalation Delivery for the Treatment and Prevention of COVID-19 Infection, Pharmaceutics
Fiolet, Kherabi, Macdonald, Etal, Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review, Clin Microbiol Infect
Gao, Ding, Dong, Risk factors for severe and critically ill COVID-19 patients: A review, Allergy
Griesel, Wagner, Mikolajewska, Inhaled corticosteroids for the treatment of COVID-19, Cochrane Database Syst Rev
Kino, Burd, Segars, Dexamethasone for Severe COVID-19: How Does It Work at Cellular and Molecular Levels?, Int J Mol Sci
Omolo, Soni, Fasiku, Mackraj, Govender, Update on therapeutic approaches and emerging therapies for SARS-CoV-2 virus, Eur J Pharmacol
Pandya, Puttanna, Balagopal, Systemic effects of inhaled corticosteroids: an overview, Open Respir Med J
Papi, Marku, Scichilone, Regular versus asneeded budesonide and formoterol combination treatment for moderate asthma: a non-inferiority, randomised, double-blind clinical trial, Lancet Respir Med
Patel, Corticosteroids for treatment of COVID-19: effect, evidence, expectation and extent, Beni-Suef University journal of basic and applied sciences
Selarka, Sharma, Saini, Mucormycosis and COVID-19: An epidemic within a pandemic in India, Mycoses
Song, Yoon, Seo, Ciclesonide Inhaler Treatment for Mild-to-Moderate COVID-19: A Randomized, Open-Label, Phase 2 Trial, J Clin Med
Wagner, Weinberger, Vaccines to Prevent Infectious Diseases in the Older Population: Immunological Challenges and Future Perspectives, Front Immunol
Wu, Mcgoogan, Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention, JAMA -J Am Med Assoc
Yamaya, Nishimura, Deng, Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells, Respir Investig
Ye, Wang, Mao, The pathogenesis and treatment of the `Cytokine Storm' in COVID-19, J Infect
Yu, Bafadhel, Dorward, Hayward, Saville et al., Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet
