Rapid Elimination of Culturable SARS-CoV-2 With Intramuscular or Intravenous Administration of Antiviral Monoclonal Antibody Therapy
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf542, ACTIV-2/A5401, NCT04518410, Aug 2025
42nd treatment shown to reduce risk in
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
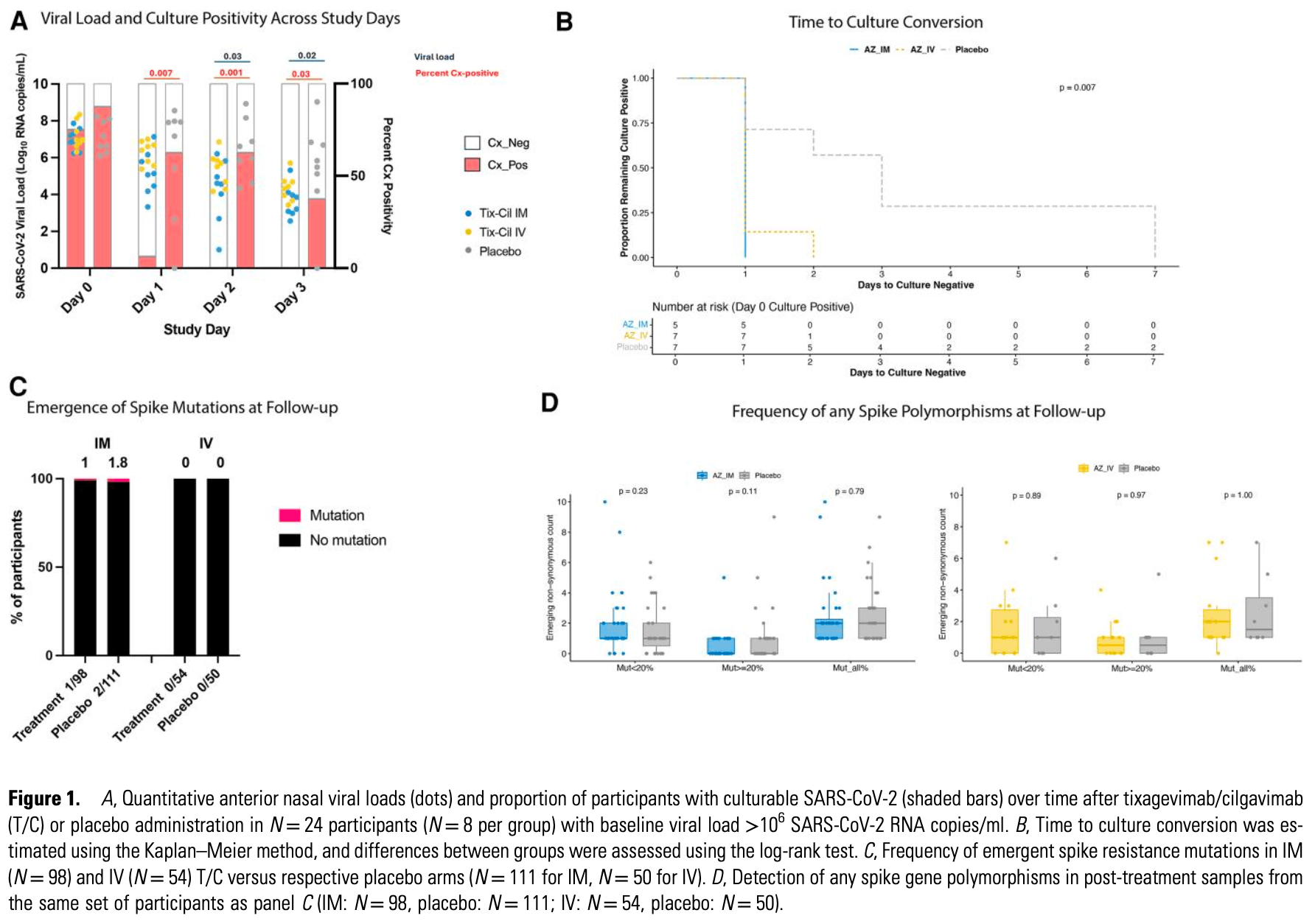
RCT 313 outpatients showing that that intramuscular delivery of tixagevimab/cilgavimab is as effective as intravenous administration for viral clearance.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2.75.2, BA.4.6, BQ.1.11, BA.5, BA.2.75, XBB2,3, XBB.1.53, ХВВ.1.9.13, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.14.
1.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
2.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Deo et al., 29 Aug 2025, Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 26 authors, trial NCT04518410 (history) (ACTIV-2/A5401).
Rapid Elimination of Culturable SARS-CoV-2 With Intramuscular or Intravenous Administration of Antiviral Monoclonal Antibody Therapy
Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf542
We evaluated intramuscular (IM) versus intravenous (IV) administration of tixagevimab/cilgavimab in early COVID-19. Both routes achieved rapid elimination of culturable virus and minimal emergence of resistance. These results support IM delivery as a viable alternative to IV, with important implications for scalable deployment in future viral pandemics.
References
Bender Ignacio, Chew, Moser, Safety and efficacy of combined tixagevimab and cilgavimab administered intramuscularly or intravenously in nonhospitalized patients with COVID-19: 2 randomized clinical trials, JAMA Netw Open
Bender Ignacio, Wohl, Arends, Comparative pharmacokinetics of tixagevimab/cilgavimab (AZD7442) administered intravenously versus intramuscularly in symptomatic SARS-CoV-2 infection, Clin Pharmacol Ther
Boeckh, Berrey, Bowden, Phase 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants, J Infect Dis
Boucau, Chew, Choudhary, Monoclonal antibody treatment drives rapid culture conversion in SARS-CoV-2 infection, Cell Rep Med
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19, N Engl J Med
Choi, Choudhary, Regan, Persistence and evolution of SARS-CoV-2 in an immunocompromised host, N Engl J Med
Choudhary, Chew, Deo, Emergence of SARS-CoV-2 escape mutations during bamlanivimab therapy in a phase II randomized clinical trial, Nat Microbiol
Choudhary, Deo, Evering, Characterization of treatment resistance and viral kinetics in the setting of single-active versus dual-active monoclonal antibodies against severe acute respiratory syndrome coronavirus 2, J Infect Dis
Dougan, Nirula, Azizad, Bamlanivimab plus etesevimab in mild or moderate COVID-19, N Engl J Med
Fenaux, Gueneau, Chaghouri, Emergence of SARS-CoV-2 resistance mutations in a patient who received anti-SARS-COV2 spike protein monoclonal antibodies: a case report, BMC Infect Dis
Gupta, Gonzalez-Rojas, Juarez, Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med
Gupta, Konnova, Smet, Host immunological responses facilitate development of SARS-CoV-2 mutations in patients receiving monoclonal antibody treatments, J Clin Invest, doi:10.1172/JCI166032
Huygens, Munnink, Gharbharan, Sotrovimab resistance and viral persistence after treatment of immunocompromised patients infected with the severe acute respiratory syndrome coronavirus 2 omicron variant, Clin Infect Dis
Jalbert, Hussein, Mastey, Effectiveness of subcutaneous casirivimab and imdevimab in ambulatory patients with COVID-19, Infect Dis Ther
Jensen, Luebke, Feldt, Emergence of the E484K mutation in SARS-COV-2-infected immunocompromised patients treated with bamlanivimab in Germany, Lancet Reg Health Eur
Kelley, Moor, Douglas, Monoclonal antibody therapies for COVID-19: lessons learned and implications for the development of future products, Curr Opin Biotechnol
Kemp, Collier, Datir, SARS-CoV-2 evolution during treatment of chronic infection, Nature
Lambrou, Redd, Stewart, Implementation of SARS-CoV-2 monoclonal antibody infusion sites at three medical centers in the United States: strengths and challenges assessment to inform COVID-19 pandemic and future public health emergency use, Disaster Med Public Health Prep
Levin, Ustianowski, Wit, Intramuscular AZD7442 (tixagevimabcilgavimab) for prevention of COVID-19, N Engl J Med
Li, Choudhary, Regan, SARS-CoV-2 viral clearance and evolution varies by type and severity of immunodeficiency, Sci Transl Med
Mulangu, Dodd, Davey, A randomized, controlled trial of ebola virus disease therapeutics, N Engl J Med
O'brien, Forleo-Neto, Musser, Subcutaneous REGEN-COV antibody combination to prevent COVID-19, N Engl J Med
Pitiot, Heuze-Vourc'h, Secher, Alternative routes of administration for therapeutic antibodies-state of the art, Antibodies
Shapiro, Sarkis, Acloque, Intramuscular vs intravenous SARS-CoV-2 neutralizing antibody sotrovimab for treatment of COVID-19 (COMET-TAIL): a randomized noninferiority clinical trial, Open Forum Infect Dis
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19, N Engl J Med
Westendorf, Žentelis, Wang, LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants, Cell Rep
DOI record:
{
"DOI": "10.1093/ofid/ofaf542",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofaf542",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>We evaluated intramuscular (IM) versus intravenous (IV) administration of tixagevimab/cilgavimab in early COVID-19. Both routes achieved rapid elimination of culturable virus and minimal emergence of resistance. These results support IM delivery as a viable alternative to IV, with important implications for scalable deployment in future viral pandemics.</jats:p>",
"article-number": "ofaf542",
"author": [
{
"affiliation": [
{
"name": "Brigham and Women’s Hospital, Harvard Medical School , Boston, Massachusetts, USA"
}
],
"family": "Deo",
"given": "Rinki",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Brigham and Women’s Hospital, Harvard Medical School , Boston, Massachusetts, USA"
}
],
"family": "Choudhary",
"given": "Manish C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ragon Institute of Mass General Brigham, MIT, and Harvard , Cambridge, Massachusetts, USA"
}
],
"family": "Glover",
"given": "Owen T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Washington , Seattle, Washington, USA"
},
{
"name": "Fred Hutch Cancer Center , Seattle, Washington, USA"
}
],
"family": "Ignacio",
"given": "Rachel Bender",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6731-7238",
"affiliation": [
{
"name": "Ragon Institute of Mass General Brigham, MIT, and Harvard , Cambridge, Massachusetts, USA"
}
],
"authenticated-orcid": false,
"family": "Boucau",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "David Geffen School of Medicine, UCLA , Los Angeles, California, USA"
}
],
"family": "Chew",
"given": "Kara W",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5601-9112",
"affiliation": [
{
"name": "Harvard T.H. Chan School of Public Health , Boston, Massachusetts, USA"
}
],
"authenticated-orcid": false,
"family": "Moser",
"given": "Carlee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "David Geffen School of Medicine, UCLA , Los Angeles, California, USA"
}
],
"family": "Currier",
"given": "Judith S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "UNC School of Medicine , Chapel Hill, North Carolina, USA"
}
],
"family": "Eron",
"given": "Joseph J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institutes of Health , Bethesda, Maryland, USA"
}
],
"family": "Javan",
"given": "Arzhang Cyrus",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard T.H. Chan School of Public Health , Boston, Massachusetts, USA"
}
],
"family": "Giganti",
"given": "Mark J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard T.H. Chan School of Public Health , Boston, Massachusetts, USA"
}
],
"family": "Aga",
"given": "Evgenia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vaccines and Immunotherapies, AstraZeneca , Cambridge ,",
"place": [
"UK"
]
}
],
"family": "Gibbs",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vaccines and Immunotherapies, AstraZeneca , Gaithersburg ,",
"place": [
"USA"
]
}
],
"family": "Cohen",
"given": "Taylor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vaccines and Immunotherapies, AstraZeneca , Gaithersburg ,",
"place": [
"USA"
]
}
],
"family": "Streicher",
"given": "Katie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vaccines and Immunotherapies, AstraZeneca , Gaithersburg ,",
"place": [
"USA"
]
}
],
"family": "Soboleva",
"given": "Karina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Nebraska Medical Center , Omaha, Nebraska, USA"
}
],
"family": "Fletcher",
"given": "Courtney V",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Lundquist Institute, Harbor-UCLA Medical Center , Los Angeles, California, USA"
}
],
"family": "Daar",
"given": "Eric S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Washington , Seattle, Washington, USA"
}
],
"family": "Greninger",
"given": "Alexander L",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Washington , Seattle, Washington, USA"
}
],
"family": "Coombs",
"given": "Robert W",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4900-098X",
"affiliation": [
{
"name": "UNC School of Medicine , Chapel Hill, North Carolina, USA"
}
],
"authenticated-orcid": false,
"family": "Fischer",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard T.H. Chan School of Public Health , Boston, Massachusetts, USA"
}
],
"family": "Hughes",
"given": "Michael D",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of California San Diego , San Diego, California, USA"
}
],
"family": "Smith",
"given": "Davey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "UNC School of Medicine , Chapel Hill, North Carolina, USA"
}
],
"family": "Wohl",
"given": "David Alain",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ragon Institute of Mass General Brigham, MIT, and Harvard , Cambridge, Massachusetts, USA"
},
{
"name": "Massachusetts General Hospital, Harvard Medical School , Boston, Massachusetts, USA"
}
],
"family": "Barczak",
"given": "Amy K",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Brigham and Women’s Hospital, Harvard Medical School , Boston, Massachusetts, USA"
}
],
"family": "Li",
"given": "Jonathan Z",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
9,
8
]
],
"date-time": "2025-09-08T10:59:56Z",
"timestamp": 1757329196000
},
"deposited": {
"date-parts": [
[
2025,
9,
25
]
],
"date-time": "2025-09-25T12:53:44Z",
"timestamp": 1758804824000
},
"funder": [
{
"DOI": "10.13039/501100012264",
"award": [
"UM1 AI068634",
"UM1 AI068636",
"UM1 AI106701"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100012264",
"id-type": "DOI"
}
],
"name": "NIH"
}
],
"indexed": {
"date-parts": [
[
2025,
9,
26
]
],
"date-time": "2025-09-26T00:18:14Z",
"timestamp": 1758845894702,
"version": "3.44.0"
},
"is-referenced-by-count": 0,
"issue": "9",
"issued": {
"date-parts": [
[
2025,
8,
29
]
]
},
"journal-issue": {
"issue": "9",
"published-print": {
"date-parts": [
[
2025,
8,
29
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 10,
"start": {
"date-parts": [
[
2025,
9,
8
]
],
"date-time": "2025-09-08T00:00:00Z",
"timestamp": 1757289600000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofaf542/64220045/ofaf542.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/12/9/ofaf542/64220045/ofaf542.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/12/9/ofaf542/64220045/ofaf542.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2025,
8,
29
]
]
},
"published-online": {
"date-parts": [
[
2025,
9,
8
]
]
},
"published-other": {
"date-parts": [
[
2025,
9
]
]
},
"published-print": {
"date-parts": [
[
2025,
8,
29
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1001/jamanetworkopen.2023.10039",
"article-title": "Safety and efficacy of combined tixagevimab and cilgavimab administered intramuscularly or intravenously in nonhospitalized patients with COVID-19: 2 randomized clinical trials",
"author": "Bender Ignacio",
"doi-asserted-by": "crossref",
"first-page": "e2310039",
"journal-title": "JAMA Netw Open",
"key": "2025092508533667600_ofaf542-B1",
"volume": "6",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "229",
"journal-title": "N Engl J Med",
"key": "2025092508533667600_ofaf542-B2",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "N Engl J Med",
"key": "2025092508533667600_ofaf542-B3",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/j.celrep.2022.110812",
"article-title": "LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants",
"author": "Westendorf",
"doi-asserted-by": "crossref",
"first-page": "110812",
"journal-title": "Cell Rep",
"key": "2025092508533667600_ofaf542-B4",
"volume": "39",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab plus etesevimab in mild or moderate COVID-19",
"author": "Dougan",
"doi-asserted-by": "crossref",
"first-page": "1382",
"journal-title": "N Engl J Med",
"key": "2025092508533667600_ofaf542-B5",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116620",
"article-title": "Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of COVID-19",
"author": "Levin",
"doi-asserted-by": "crossref",
"first-page": "2188",
"journal-title": "N Engl J Med",
"key": "2025092508533667600_ofaf542-B6",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "2025092508533667600_ofaf542-B7",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03291-y",
"article-title": "SARS-CoV-2 evolution during treatment of chronic infection",
"author": "Kemp",
"doi-asserted-by": "crossref",
"first-page": "277",
"journal-title": "Nature",
"key": "2025092508533667600_ofaf542-B8",
"volume": "592",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06902-1",
"article-title": "Emergence of SARS-CoV-2 resistance mutations in a patient who received anti-SARS-COV2 spike protein monoclonal antibodies: a case report",
"author": "Fenaux",
"doi-asserted-by": "crossref",
"first-page": "1223",
"journal-title": "BMC Infect Dis",
"key": "2025092508533667600_ofaf542-B9",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.lanepe.2021.100164",
"article-title": "Emergence of the E484K mutation in SARS-COV-2-infected immunocompromised patients treated with bamlanivimab in Germany",
"author": "Jensen",
"doi-asserted-by": "crossref",
"first-page": "100164",
"journal-title": "Lancet Reg Health Eur",
"key": "2025092508533667600_ofaf542-B10",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciac601",
"article-title": "Sotrovimab resistance and viral persistence after treatment of immunocompromised patients infected with the severe acute respiratory syndrome coronavirus 2 omicron variant",
"author": "Huygens",
"doi-asserted-by": "crossref",
"first-page": "e507",
"journal-title": "Clin Infect Dis",
"key": "2025092508533667600_ofaf542-B11",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1172/JCI166032",
"article-title": "Host immunological responses facilitate development of SARS-CoV-2 mutations in patients receiving monoclonal antibody treatments",
"author": "Gupta",
"doi-asserted-by": "publisher",
"first-page": "e166032",
"journal-title": "J Clin Invest",
"key": "2025092508533667600_ofaf542-B12",
"volume": "133",
"year": "2023"
},
{
"DOI": "10.1056/NEJMc2031364",
"article-title": "Persistence and evolution of SARS-CoV-2 in an immunocompromised host",
"author": "Choi",
"doi-asserted-by": "crossref",
"first-page": "2291",
"journal-title": "N Engl J Med",
"key": "2025092508533667600_ofaf542-B13",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1126/scitranslmed.adk1599",
"article-title": "SARS-CoV-2 viral clearance and evolution varies by type and severity of immunodeficiency",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "eadk1599",
"journal-title": "Sci Transl Med",
"key": "2025092508533667600_ofaf542-B14",
"volume": "16",
"year": "2024"
},
{
"DOI": "10.1086/322043",
"article-title": "Phase 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants",
"author": "Boeckh",
"doi-asserted-by": "crossref",
"first-page": "350",
"journal-title": "J Infect Dis",
"key": "2025092508533667600_ofaf542-B15",
"volume": "184",
"year": "2001"
},
{
"DOI": "10.1056/NEJMoa1910993",
"article-title": "A randomized, controlled trial of ebola virus disease therapeutics",
"author": "Mulangu",
"doi-asserted-by": "crossref",
"first-page": "2293",
"journal-title": "N Engl J Med",
"key": "2025092508533667600_ofaf542-B16",
"volume": "381",
"year": "2019"
},
{
"DOI": "10.1002/cpt.2706",
"article-title": "Comparative pharmacokinetics of tixagevimab/cilgavimab (AZD7442) administered intravenously versus intramuscularly in symptomatic SARS-CoV-2 infection",
"author": "Bender Ignacio",
"doi-asserted-by": "crossref",
"first-page": "1207",
"journal-title": "Clin Pharmacol Ther",
"key": "2025092508533667600_ofaf542-B17",
"volume": "112",
"year": "2022"
},
{
"DOI": "10.1093/ofid/ofad354",
"article-title": "Intramuscular vs intravenous SARS-CoV-2 neutralizing antibody sotrovimab for treatment of COVID-19 (COMET-TAIL): a randomized noninferiority clinical trial",
"author": "Shapiro",
"doi-asserted-by": "crossref",
"first-page": "ofad354",
"journal-title": "Open Forum Infect Dis",
"key": "2025092508533667600_ofaf542-B18",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1007/s40121-022-00691-z",
"article-title": "Effectiveness of subcutaneous casirivimab and imdevimab in ambulatory patients with COVID-19",
"author": "Jalbert",
"doi-asserted-by": "crossref",
"first-page": "2125",
"journal-title": "Infect Dis Ther",
"key": "2025092508533667600_ofaf542-B19",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2109682",
"article-title": "Subcutaneous REGEN-COV antibody combination to prevent COVID-19",
"author": "O’Brien",
"doi-asserted-by": "crossref",
"first-page": "1184",
"journal-title": "N Engl J Med",
"key": "2025092508533667600_ofaf542-B20",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/j.xcrm.2022.100678",
"article-title": "Monoclonal antibody treatment drives rapid culture conversion in SARS-CoV-2 infection",
"author": "Boucau",
"doi-asserted-by": "crossref",
"first-page": "100678",
"journal-title": "Cell Rep Med",
"key": "2025092508533667600_ofaf542-B21",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1017/dmp.2022.15",
"article-title": "Implementation of SARS-CoV-2 monoclonal antibody infusion sites at three medical centers in the United States: strengths and challenges assessment to inform COVID-19 pandemic and future public health emergency use",
"author": "Lambrou",
"doi-asserted-by": "crossref",
"first-page": "e112",
"journal-title": "Disaster Med Public Health Prep",
"key": "2025092508533667600_ofaf542-B22",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiae192",
"article-title": "Characterization of treatment resistance and viral kinetics in the setting of single-active versus dual-active monoclonal antibodies against severe acute respiratory syndrome coronavirus 2",
"author": "Choudhary",
"doi-asserted-by": "crossref",
"first-page": "394",
"journal-title": "J Infect Dis",
"key": "2025092508533667600_ofaf542-B23",
"volume": "230",
"year": "2024"
},
{
"DOI": "10.1038/s41564-022-01254-1",
"article-title": "Emergence of SARS-CoV-2 escape mutations during bamlanivimab therapy in a phase II randomized clinical trial",
"author": "Choudhary",
"doi-asserted-by": "crossref",
"first-page": "1906",
"journal-title": "Nat Microbiol",
"key": "2025092508533667600_ofaf542-B24",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1016/j.copbio.2022.102798",
"article-title": "Monoclonal antibody therapies for COVID-19: lessons learned and implications for the development of future products",
"author": "Kelley",
"doi-asserted-by": "crossref",
"first-page": "102798",
"journal-title": "Curr Opin Biotechnol",
"key": "2025092508533667600_ofaf542-B25",
"volume": "78",
"year": "2022"
},
{
"DOI": "10.3390/antib11030056",
"article-title": "Alternative routes of administration for therapeutic antibodies-state of the art",
"author": "Pitiot",
"doi-asserted-by": "crossref",
"first-page": "56",
"journal-title": "Antibodies (Basel)",
"key": "2025092508533667600_ofaf542-B26",
"volume": "11",
"year": "2022"
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofaf542/8249030"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Rapid Elimination of Culturable SARS-CoV-2 With Intramuscular or Intravenous Administration of Antiviral Monoclonal Antibody Therapy",
"type": "journal-article",
"volume": "12"
}
