Anti-Granulocyte–Macrophage Colony–Stimulating Factor Monoclonal Antibody Gimsilumab for COVID-19 Pneumonia: A Randomized, Double-Blind, Placebo-controlled Trial
et al., American Journal of Respiratory and Critical Care Medicine, doi:10.1164/rccm.202108-1859OC, BREATHE, NCT04351243, Jun 2022
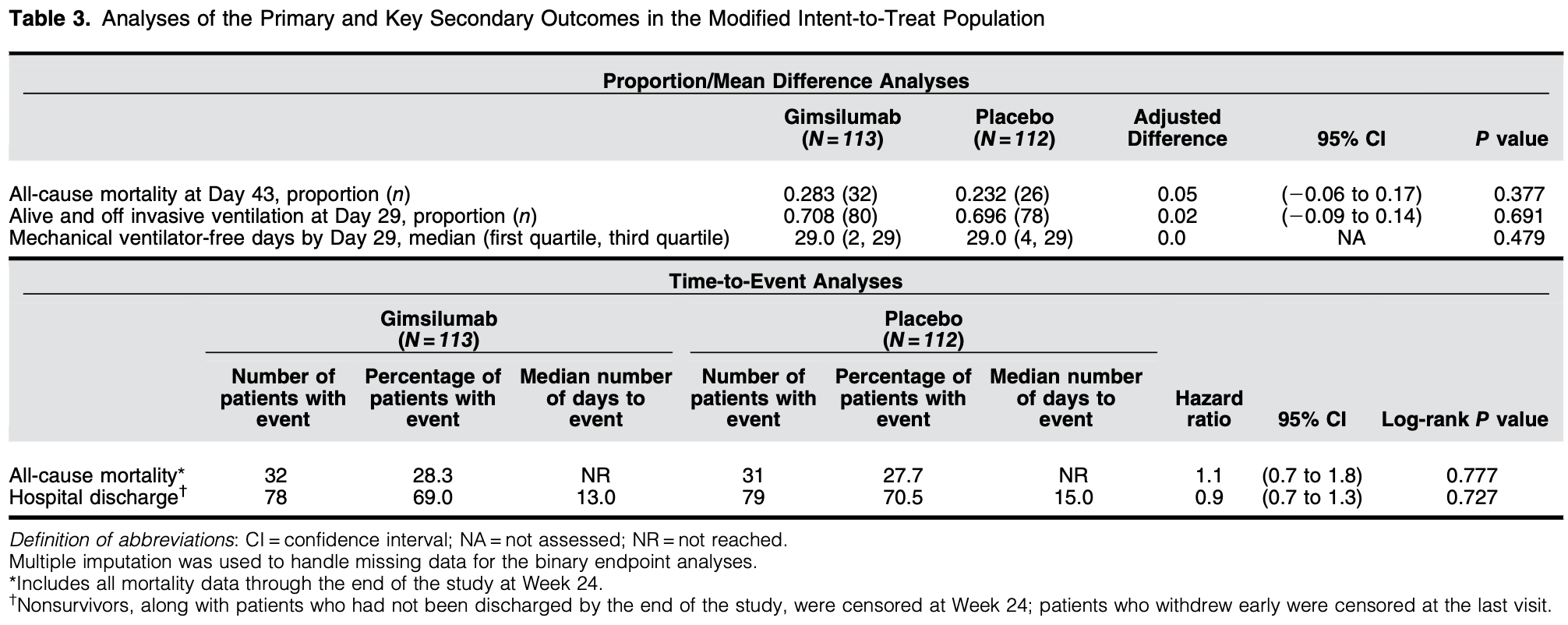
RCT 225 hospitalized COVID-19 patients showing no significant difference in mortality or clinical outcomes with the anti-GM-CSF monoclonal antibody gimsilumab compared to placebo.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
risk of death, 10.0% higher, HR 1.10, p = 0.71, treatment 32 of 113 (28.3%), control 31 of 112 (27.7%), adjusted per study, day 168.
|
|
risk of death, 22.0% higher, RR 1.22, p = 0.45, treatment 32 of 113 (28.3%), control 26 of 112 (23.2%), day 43.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Criner et al., 1 Jun 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 23 authors, study period 15 April, 2020 - 12 October, 2020, average treatment delay 10.0 days, trial NCT04351243 (history) (BREATHE).
Contact: dgern@thoracic.org, simon.lowry@kinevant.com.
Anti-Granulocyte-Macrophage Colony-Stimulating Factor Monoclonal Antibody Gimsilumab for COVID-19 Pneumonia A Randomized, Double-Blind, Placebo-controlled Trial
doi:10.1164/rccm.202108-1859OCon
Rationale: GM-CSF (granulocyte-macrophage colony-stimulating factor) has emerged as a promising target against the hyperactive host immune response associated with coronavirus disease . Objectives: We sought to investigate the efficacy and safety of gimsilumab, an anti-GM-CSF monoclonal antibody, for the treatment of hospitalized patients with elevated inflammatory markers and hypoxemia secondary to COVID-19. Methods: We conducted a 24-week randomized, double-blind, placebo-controlled trial, BREATHE, at 21 locations in the United States. Patients were randomized 1:1 to receive two doses of intravenous gimsilumab or placebo 1 week apart. The primary endpoint was all-cause mortality rate at Day 43. Key secondary outcomes were ventilator-free survival rate, ventilator-free days, and time to hospital discharge. Enrollment was halted early for futility based on an interim analysis. Measurements and Main Results: Of the planned 270 patients, 225 were randomized and dosed; 44.9% of patients were Hispanic or Latino. The gimsilumab and placebo groups experienced an allcause mortality rate at Day 43 of 28.3% and 23.2%, respectively (adjusted difference = 5% vs. placebo; 95% confidence interval [26 to 17]; P = 0.377). Overall mortality rates at 24 weeks were similar across the treatment arms. The key secondary endpoints demonstrated no significant differences between groups. Despite the high background use of corticosteroids and anticoagulants, adverse events were generally balanced between treatment groups. Conclusions: Gimsilumab did not improve mortality or other key clinical outcomes in patients with COVID-19 pneumonia and evidence of systemic inflammation. The utility of anti-GM-CSF therapy for COVID-19 remains unclear. Clinical trial registered with www.clinicaltrials.gov (NCT 04351243).
Author disclosures are available with the text of this article at www.atsjournals.org.
Acknowledgment: The authors thank the patients and caregivers for their participation in the BREATHE study. The authors thank all site investigators and staff for their support in conducting the study. The authors thank the contract research organization Parexel for its important role. The authors continue to be inspired by the courage and resilience of the medical community and society at large in the face of this global pandemic.
References
Bonaventura, Vecchi E A, Wang, Lee, Cremer et al., Targeting GM-CSF in COVID-19 pneumonia: rationale and strategies, Front Immunol
Brown, Duggal, Hou, Tidswell, Khan et al., National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) Prevention and Early Treatment of Acute Lung Injury (PETAL) Network. Nonlinear imputation of PaO2/FI O 2 From Sp O 2 /FI O 2 among mechanically ventilated patients in the ICU: a prospective, observational study, Crit Care Med
Brown, Grissom, Moss, Rice, Schoenfeld et al., Nonlinear imputation of Pao2/Fi o 2 from Sp o 2 /Fi o 2 among patients with acute respiratory distress syndrome, Chest
Calfee, Delucchi, Sinha, Matthay, Hackett et al., Irish Critical Care Trials Group. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial, Lancet Respir Med
Caricchio, Abbate, Gordeev, Meng, Hsue et al., Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial, JAMA
Caricchio, Gallucci, Dass, Zhang, Gallucci et al., Temple University COVID-19 Research Group. Preliminary predictive criteria for COVID-19 cytokine storm, Ann Rheum Dis
Chen, Xie, Su, Wang, Sun et al., Corticosteroid therapy is associated with improved outcome in critically ill patients with COVID-19 with hyperinflammatory phenotype, Chest
Chertoff, High-flow oxygen, positive end-expiratory pressure, and the berlin definition of acute respiratory distress syndrome: are they mutually exclusive?, Am J Respir Crit Care Med
Cid, Unizony, Pupim, Fang, Pirrello et al., Mavrilimumab (anti GM-CSF receptor a monoclonal antibody) reduces time to flare and increases sustained remission in a phase 2 trial of patients with giant cell arteritis [abstract, Arthritis Rheumatol
De Alessandris, Ferguson, Dodd, Juss, Devaprasad et al., Neutrophil GM-CSF receptor dynamics in acute lung injury, J Leukoc Biol
De Luca, Cavalli, Campochiaro, Della-Torre, Angelillo et al., GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study, Lancet Rheumatol
Fajgenbaum, June, Cytokine storm, N Engl J Med
Fisher, Veenith, Slade, Gaskell, Rowland et al., Namilumab or infliximab compared with standard of care in hospitalised patients with COVID-19 (CATALYST): a randomised, multicentre, multi-arm, multistage, open-label, adaptive, phase 2, proof-of-concept trial, Lancet Respir Med
Gottlieb, Lang, Criner, Anti-GM-CSF monoclonal antibody gimsilumab improved ventilator-free survival, decreased hospitalization length, and prevented NT-proBNP rise in invasively ventilated patients with hyperinflammatory COVID-19 pneumonia: a subgroup analysis from the BREATHE trial suggests neurohormonal role for GM-CSF inhibition, Circulation
Hamilton, GM-CSF in inflammation, J Exp Med
Humanigen, FDA has declined humanigen's emergency use authorization (EUA) request for lenzilumab in hospitalized COVID-19 patients
Khameneh, Isa, Min, Nih, Ruedl, GM-CSF signalling boosts dramatically IL-1 production, PLoS One
Lang, Lee, Teijaro, Becher, Hamilton, GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches, Nat Rev Immunol
Liao, Liu, Yuan, Wen, Xu et al., Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19, Nat Med
Matthay, Thompson, Ware, The Berlin definition of acute respiratory distress syndrome: should patients receiving high-flow nasal oxygen be included?, Lancet Respir Med
Mehta, Porter, Manson, Isaacs, Openshaw et al., Therapeutic blockade of granulocyte macrophage colonystimulating factor in COVID-19-associated hyperinflammation: challenges and opportunities, Lancet Respir Med
Merad, Martin, Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages, Nat Rev Immunol
Patel, Beishuizen, Ruiz, A randomized trial of otilimab in severe COVID-19 pneumonia (OSCAR), doi:10.1101/2021.04.14.21255475v1.full.pdf
Puljic, Benediktus, Plater-Zyberk, Baeuerle, Szelenyi et al., Lipopolysaccharide-induced lung inflammation is inhibited by neutralization of GM-CSF, Eur J Pharmacol
Pupim, Wang, Hudock, Mavrilimumab improves outcomes in phase 2 trial in non-mechanically-ventilated patients with severe COVID-19 pneumonia and systemic hyperinflammation, Ann Rheum Dis
Ranieri, Rubenfeld, Thompson, Ferguson, ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition, JAMA
Ranjeva, Pinciroli, Hodell, Mueller, Hardin et al., Identifying clinical and biochemical phenotypes in acute respiratory distress syndrome secondary to coronavirus disease-2019, EClinicalMedicine
Shankar-Hari, Vale, Godolphin, Fisher, Higgins et al., WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis, JAMA
Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal, J Heart Lung Transplant
Sinha, Delucchi, Thompson, Mcauley, Matthay et al., Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study, Intensive Care Med
Sterner, Sakemura, Cox, Yang, Khadka et al., GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts, Blood
Stone, Frigault, Serling-Boyd, Fernandes, Harvey et al., Efficacy of tocilizumab in patients hospitalized with Covid-19, N Engl J Med
Temesgen, Assi, Shweta, Vergidis, Rizza et al., GM-CSF neutralization with lenzilumab in severe COVID-19 pneumonia: a case-cohort study, Mayo Clin Proc
Temesgen, Burger, Baker, Polk, Libertin et al., Lenzilumab in hospitalised patients with COVID-19 pneumonia (LIVE-AIR): a phase 3, randomised, placebo-controlled trial, Lancet Respir Med
Thwaites, Sevilla Uruchurtu, Siggins, Liew, Russell et al., ISARIC4C investigators. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19, Sci Immunol
Tugues, Amorim, Spath, Martin-Blondel, Schreiner et al., Graft-versus-host disease, but not graft-versus-leukemia immunity, is mediated by GM-CSF-licensed myeloid cells, Sci Transl Med
Vincent, Moreno, Takala, Willatts, Mendonc ¸a et al., The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine, Intensive Care Med
Wu, Mcgoogan, Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, JAMA
Zhou, Fu, Zheng, Wang, Zhao et al., Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients, Natl Sci Rev
DOI record:
{
"DOI": "10.1164/rccm.202108-1859oc",
"ISSN": [
"1073-449X",
"1535-4970"
],
"URL": "http://dx.doi.org/10.1164/rccm.202108-1859OC",
"alternative-id": [
"10.1164/rccm.202108-1859OC"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-08-08"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2022-03-15"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2022-06-01"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Thoracic Medicine and Surgery, Lewis Katz School of Medicine, Temple University, Philadelphia, Pennsylvania;"
}
],
"family": "Criner",
"given": "Gerard J.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-8866-4490",
"affiliation": [
{
"name": "Roivant Sciences, New York, New York;"
},
{
"name": "Kinevant Sciences, a wholly-owned subsidiary of Roivant Sciences, New York, New York;"
},
{
"name": "Columbia University Vagelos College of Physicians and Surgeons, New York, New York;"
}
],
"authenticated-orcid": false,
"family": "Lang",
"given": "Frederick M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8376-8709",
"affiliation": [
{
"name": "Baylor University Medical Center, Dallas, Texas;"
},
{
"name": "Baylor Scott & White The Heart Hospital–Plano, Plano, Texas;"
},
{
"name": "Baylor Scott & White Heart and Vascular Hospital, Dallas, Texas;"
}
],
"authenticated-orcid": false,
"family": "Gottlieb",
"given": "Robert L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Icahn School of Medicine at Mount Sinai, New York, New York;"
}
],
"family": "Mathews",
"given": "Kusum S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of California Los Angeles David Geffen School of Medicine, Los Angeles, California;"
}
],
"family": "Wang",
"given": "Tisha S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vanderbilt University Medical Center, Nashville, Tennessee;"
}
],
"family": "Rice",
"given": "Todd W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Icahn School of Medicine at Mount Sinai, New York, New York;"
}
],
"family": "Madduri",
"given": "Deepu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "NorthShore University HealthSystem, Evanston, Illinois;"
}
],
"family": "Bellam",
"given": "Shashi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "East Jefferson General Hospital, Metaire, Lousiana;"
}
],
"family": "Jeanfreau",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Piedmont Healthcare, Atlanta, Georgia;"
}
],
"family": "Case",
"given": "Amy H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Arizona College of Medicine/Banner University Medical Center, Phoenix, Arizona;"
}
],
"family": "Glassberg",
"given": "Marilyn K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Emory University School of Medicine, Atlanta, Georgia;"
}
],
"family": "Lyon",
"given": "George Marshall",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Inova Fairfax Hospital, Falls Church, Virginia;"
}
],
"family": "Ahmad",
"given": "Kareem",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Jamaica Hospital Medical Center, Jamaica, New York;"
}
],
"family": "Mendelson",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Baylor Scott & White The Heart Hospital–Plano, Plano, Texas;"
}
],
"family": "DiMaio",
"given": "J. Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Baylor Scott & White Medical Center–Round Rock, Round Rock, Texas;"
}
],
"family": "Tran",
"given": "MaryAnn P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Baylor University Medical Center, Dallas, Texas;"
},
{
"name": "Texas Centers for Infectious Disease Associates, Dallas, Texas;"
}
],
"family": "Spak",
"given": "Cedric W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Baylor Scott & White All Saints Medical Center, Fort Worth, Texas;"
}
],
"family": "Abbasi",
"given": "Jamil A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Baylor Scott & White Medical Center–Irving, Irving, Texas;"
}
],
"family": "Davis",
"given": "Steven G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Baylor Scott & White Medical Center–Temple, Temple, Texas; and"
}
],
"family": "Ghamande",
"given": "Shekhar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Roivant Sciences, New York, New York;"
},
{
"name": "Kinevant Sciences, a wholly-owned subsidiary of Roivant Sciences, New York, New York;"
},
{
"name": "Sumitovant Biopharma, New York, New York"
}
],
"family": "Shen",
"given": "Steven",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Roivant Sciences, New York, New York;"
},
{
"name": "Kinevant Sciences, a wholly-owned subsidiary of Roivant Sciences, New York, New York;"
}
],
"family": "Sherman",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Roivant Sciences, New York, New York;"
},
{
"name": "Kinevant Sciences, a wholly-owned subsidiary of Roivant Sciences, New York, New York;"
}
],
"family": "Lowry",
"given": "Simon",
"sequence": "additional"
}
],
"container-title": "American Journal of Respiratory and Critical Care Medicine",
"container-title-short": "Am J Respir Crit Care Med",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.atsjournals.org"
]
},
"created": {
"date-parts": [
[
2022,
3,
15
]
],
"date-time": "2022-03-15T17:41:05Z",
"timestamp": 1647366065000
},
"deposited": {
"date-parts": [
[
2024,
9,
20
]
],
"date-time": "2024-09-20T09:34:18Z",
"timestamp": 1726824858000
},
"funder": [
{
"name": "Kinevant Sciences"
}
],
"indexed": {
"date-parts": [
[
2025,
4,
15
]
],
"date-time": "2025-04-15T16:29:04Z",
"timestamp": 1744734544019
},
"is-referenced-by-count": 28,
"issue": "11",
"issued": {
"date-parts": [
[
2022,
6,
1
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2022,
6,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.atsjournals.org/doi/pdf/10.1164/rccm.202108-1859OC",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "19",
"original-title": [],
"page": "1290-1299",
"prefix": "10.1164",
"published": {
"date-parts": [
[
2022,
6,
1
]
]
},
"published-print": {
"date-parts": [
[
2022,
6,
1
]
]
},
"publisher": "American Thoracic Society",
"reference": [
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "bib1"
},
{
"DOI": "10.1038/s41577-020-0331-4",
"doi-asserted-by": "publisher",
"key": "bib2"
},
{
"DOI": "10.1038/s41591-020-0901-9",
"doi-asserted-by": "publisher",
"key": "bib3"
},
{
"DOI": "10.1016/j.healun.2020.03.012",
"doi-asserted-by": "publisher",
"key": "bib4"
},
{
"DOI": "10.1056/NEJMra2026131",
"doi-asserted-by": "publisher",
"key": "bib5"
},
{
"DOI": "10.1038/s41577-020-0357-7",
"doi-asserted-by": "publisher",
"key": "bib6"
},
{
"DOI": "10.1016/S2213-2600(20)30267-8",
"doi-asserted-by": "publisher",
"key": "bib7"
},
{
"DOI": "10.3389/fimmu.2020.01625",
"doi-asserted-by": "publisher",
"key": "bib8"
},
{
"DOI": "10.1093/nsr/nwaa041",
"doi-asserted-by": "publisher",
"key": "bib9"
},
{
"DOI": "10.1126/sciimmunol.abg9873",
"doi-asserted-by": "publisher",
"key": "bib10"
},
{
"DOI": "10.1084/jem.20190945",
"doi-asserted-by": "publisher",
"key": "bib11"
},
{
"author": "Cid MC",
"first-page": "L06",
"journal-title": "Arthritis Rheumatol",
"key": "bib12",
"volume": "72",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(20)30170-3",
"doi-asserted-by": "publisher",
"key": "bib13"
},
{
"DOI": "10.1016/j.mayocp.2020.08.038",
"doi-asserted-by": "publisher",
"key": "bib14"
},
{
"DOI": "10.1182/blood-2018-10-881722",
"doi-asserted-by": "publisher",
"key": "bib15"
},
{
"DOI": "10.1126/scitranslmed.aat8410",
"doi-asserted-by": "publisher",
"key": "bib16"
},
{
"DOI": "10.1371/journal.pone.0023025",
"doi-asserted-by": "publisher",
"key": "bib17"
},
{
"DOI": "10.1016/j.ejphar.2006.11.023",
"doi-asserted-by": "publisher",
"key": "bib18"
},
{
"DOI": "10.1002/JLB.3MA0918-347R",
"doi-asserted-by": "publisher",
"key": "bib19"
},
{
"DOI": "10.1161/circ.144.suppl_1.14310",
"author": "Gottlieb RL",
"doi-asserted-by": "crossref",
"first-page": "A14310",
"journal-title": "Circulation",
"key": "bib20",
"volume": "144",
"year": "2021"
},
{
"DOI": "10.1016/j.chest.2016.01.003",
"doi-asserted-by": "publisher",
"key": "bib22"
},
{
"DOI": "10.1097/CCM.0000000000002514",
"doi-asserted-by": "publisher",
"key": "bib23"
},
{
"DOI": "10.1001/jama.2012.5669",
"doi-asserted-by": "publisher",
"key": "bib24"
},
{
"DOI": "10.1164/rccm.201701-0005LE",
"doi-asserted-by": "publisher",
"key": "bib25"
},
{
"DOI": "10.1016/S2213-2600(21)00105-3",
"doi-asserted-by": "publisher",
"key": "bib26"
},
{
"author": "Royal College of Physicians",
"key": "bib28",
"volume-title": "National Early Warning Score (NEWS) - standardising the assessment of acute-illness severity in the NHS.",
"year": "2012"
},
{
"DOI": "10.1007/BF01709751",
"doi-asserted-by": "publisher",
"key": "bib29"
},
{
"DOI": "10.1136/annrheumdis-2020-218323",
"doi-asserted-by": "publisher",
"key": "bib30"
},
{
"DOI": "10.1016/S2213-2600(18)30177-2",
"doi-asserted-by": "publisher",
"key": "bib31"
},
{
"DOI": "10.1007/s00134-018-5378-3",
"doi-asserted-by": "publisher",
"key": "bib32"
},
{
"DOI": "10.1016/j.eclinm.2021.100829",
"doi-asserted-by": "publisher",
"key": "bib33"
},
{
"DOI": "10.1136/annrheumdis-2021-eular.5012",
"doi-asserted-by": "publisher",
"key": "bib35"
},
{
"DOI": "10.1016/S2213-2600(21)00494-X",
"doi-asserted-by": "publisher",
"key": "bib36"
},
{
"DOI": "10.1016/S2213-2600(21)00460-4",
"doi-asserted-by": "publisher",
"key": "bib37"
},
{
"DOI": "10.1056/NEJMoa2028836",
"doi-asserted-by": "publisher",
"key": "bib38"
},
{
"DOI": "10.1001/jama.2021.9508",
"doi-asserted-by": "publisher",
"key": "bib39"
},
{
"DOI": "10.1016/j.chest.2020.11.050",
"doi-asserted-by": "publisher",
"key": "bib40"
},
{
"author": "RECOVERY Collaborative Group",
"first-page": "133",
"journal-title": "N Engl J Med",
"key": "bib41",
"year": "2020"
},
{
"DOI": "10.1001/jama.2021.11330",
"doi-asserted-by": "publisher",
"key": "bib43"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.atsjournals.org/doi/10.1164/rccm.202108-1859OC"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Anti-Granulocyte–Macrophage Colony–Stimulating Factor Monoclonal Antibody Gimsilumab for COVID-19 Pneumonia: A Randomized, Double-Blind, Placebo-controlled Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1164/crossmarkpolicies",
"updated-by": [
{
"DOI": "10.1164/rccm.v206erratum9",
"label": "Correction",
"source": "publisher",
"type": "correction",
"updated": {
"date-parts": [
[
2022,
9,
16
]
],
"date-time": "2022-09-16T00:00:00Z",
"timestamp": 1663286400000
}
}
],
"volume": "205"
}