Jun 1 2022 |
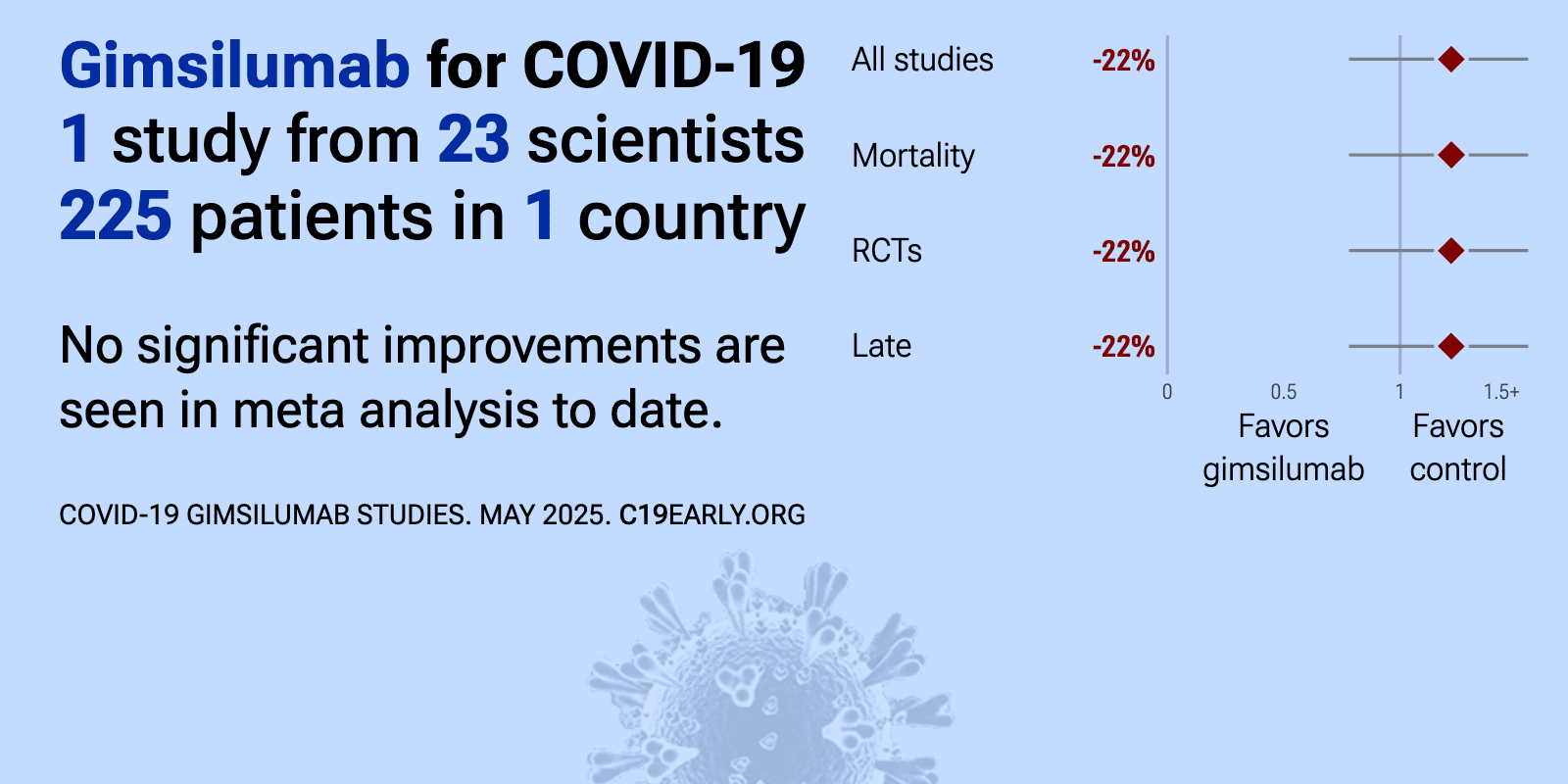
et al., American Journal of Respiratory and Critical Care Medicine, doi:10.1164/rccm.202108-1859OC | Anti-Granulocyte–Macrophage Colony–Stimulating Factor Monoclonal Antibody Gimsilumab for COVID-19 Pneumonia: A Randomized, Double-Blind, Placebo-controlled Trial |
| 10% higher mortality (p=0.71). RCT 225 hospitalized COVID-19 patients showing no significant difference in mortality or clinical outcomes with the anti-GM-CSF monoclonal antibody gimsilumab compared to placebo. | ||