Effect of phthalocyanine oral and nasal antiseptic solutions on the infectivity of SARS-CoV-2 in patients with COVID-19: a randomized controlled trial
et al., German Medical Science GMS Publishing House, doi:10.3205/000321, Jun 2023
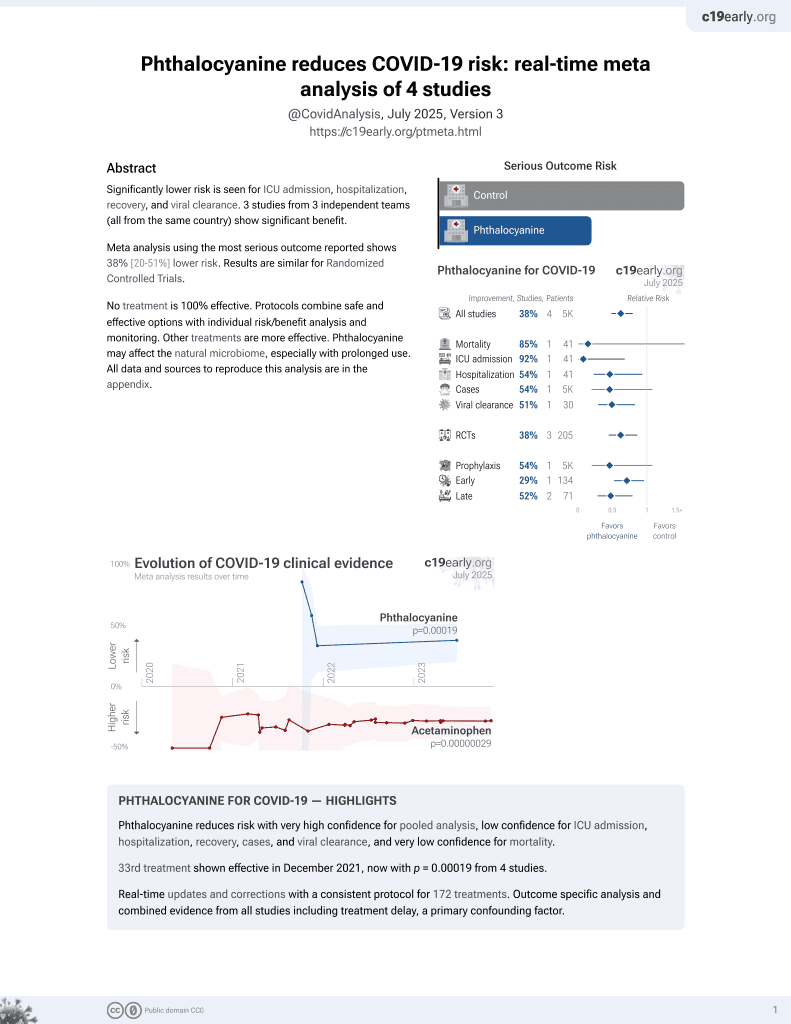
34th treatment shown to reduce risk in
December 2021, now with p = 0.00019 from 4 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
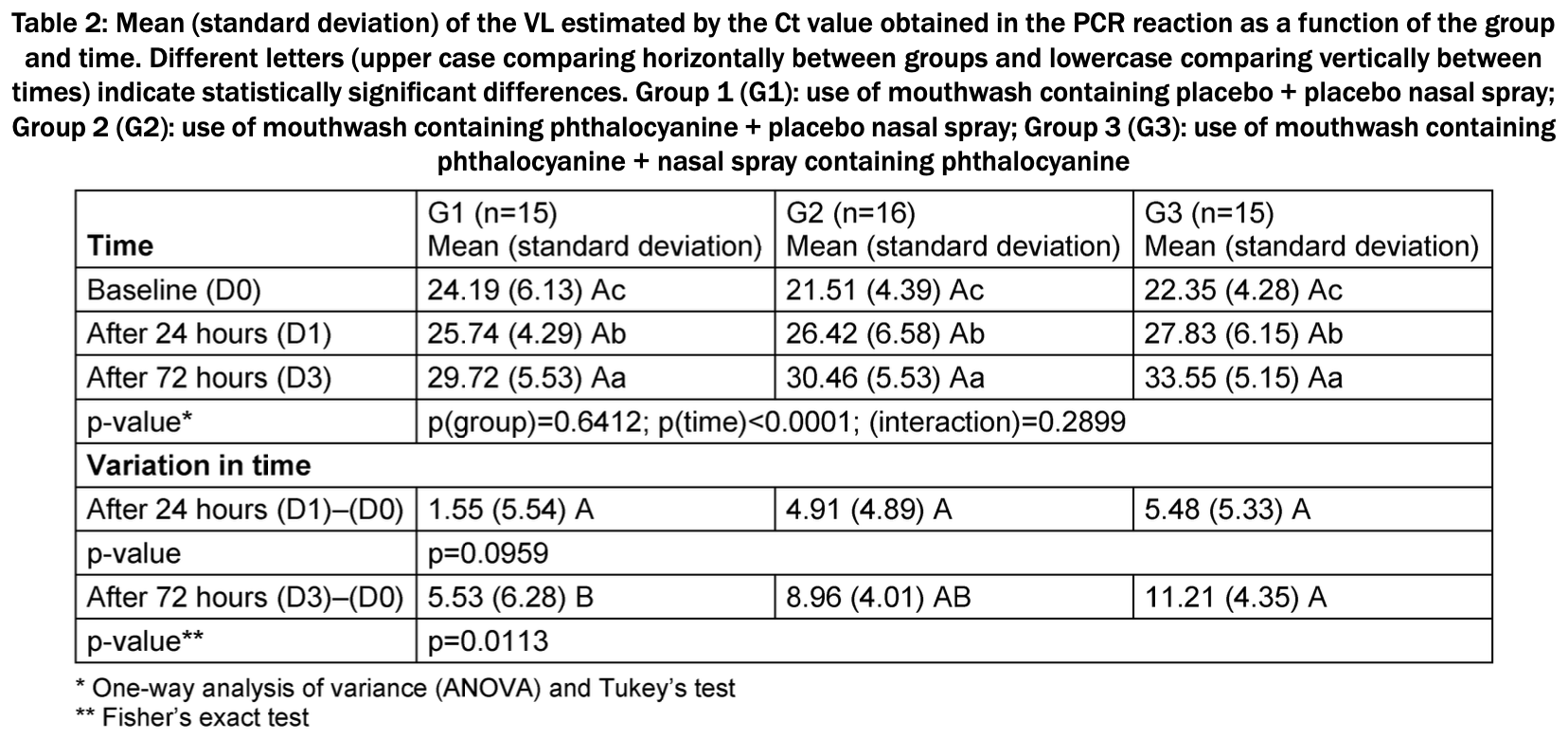
RCT 75 patients in Brazil, showing significantly lower viral load with phthalocyanine mouthwash and nasal spray. The combination was more effective than mouthwash alone.
|
relative Ct improvement, 50.7% better, RR 0.49, p = 0.008, treatment mean 11.21 (±4.35) n=15, control mean 5.53 (±6.28) n=15, mouthwash and nasal spray, day 3.
|
|
relative Ct improvement, 38.3% better, RR 0.62, p = 0.08, treatment mean 8.96 (±4.01) n=16, control mean 5.53 (±6.28) n=15, mouthwash only, day 3.
|
|
relative Ct improvement, 71.7% better, RR 0.28, p = 0.06, treatment mean 5.48 (±5.33) n=15, control mean 1.55 (±5.54) n=15, mouthwash and nasal spray, day 1.
|
|
relative Ct improvement, 68.4% better, RR 0.32, p = 0.08, treatment mean 4.91 (±4.89) n=16, control mean 1.55 (±5.54) n=15, mouthwash only, day 1.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Colado Simão et al., 23 Jun 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Brazil, peer-reviewed, 13 authors, study period 1 November, 2020 - 1 February, 2021, average treatment delay 5.4 days.
Effect of phthalocyanine oral and nasal antiseptic solutions on the infectivity of SARS-CoV-2 in patients with COVID-19: a randomized controlled trial Wirkung von oralen und nasalen antiseptischen Phthalocyanin-Lösungen auf die Infektiosität von SARS-CoV-2 bei Patienten mit COVID-19: eine randomisierte kontrollierte Studie
doi:10.3205/000321
Background: In individuals with coronavirus disease (COVID-19), the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load (VL) plays an important role in infectivity.
Ethics statement The
References
Burton, Clarkson, Goulao, Glenny, Mcbain et al., Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID-19 infection, Cochrane Database Syst Rev, doi:10.1002/14651858.CD013626.pub2
Corman, Landt, Kaiser, Molenkamp, Meijer et al., Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR, Euro Surveill, doi:10.2807/1560-7917.ES.2020.25.3.2000045
Da Fonseca Orcina, Vilhena, De Oliveira, Da, Alves et al., A Phthalocyanine Derivate Mouthwash to Gargling/Rinsing as an Option to Reduce Clinical Symptoms of COVID-19: Case Series, Clin Cosmet Investig Dent, doi:10.2147/CCIDE.S295423
Da, Santos, Da Fonseca Orcina, Da, Alves et al., A Recommendation of PHTALOX® Mouthwash for Preventing Infection and Progression of COVID-19, Acta Scient Dent Sci, doi:10.31080/ASDS.2020.04.0991
Da, Santos, Da Fonseca Orcina, Machado, Vilhena et al., Beneficial effects of a mouthwash containing an antiviral phthalocyanine derivative on the length of hospital stay for COVID-19: randomised trial, Sci Rep, doi:10.1038/s41598-021-99013-5
Davidson, Tapson, Irwin, French, Elliott et al., Pharyngeal Antisepsis to Reduce COVID-19 Pneumonia, Am J Med, doi:10.1016/j.amjmed.2020.12.001
Fajnzylber, Regan, Coxen, Corry, Wong et al., SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat Commun, doi:10.1038/s41467-020-19057-5
Fini, Oral saliva and COVID-19. Oral Oncol, doi:10.1016/j.oraloncology.2020.104821
Health, Understanding cycle threshold (Ct) in SARS-CoV-2 RT-PCR: a guide for health protection teams
Herrera, Serrano, Roldán, Sanz, Is the oral cavity relevant in SARS-CoV-2 pandemic?, Clin Oral Investig, doi:10.1007/s00784-020-03413-2
Huang, Pérez, Kato, Mikami, Okuda et al., NIH COVID-19 Autopsy Consortium; HCA Oral and Craniofacial Biological Network. SARS-CoV-2 infection of the oral cavity and saliva, Nat Med, doi:10.1038/s41591-021-01296-8
Jaafar, Aherfi, Wurtz, Grimaldier, Hoang et al., Correlation Between 3,790 Quantitative Polymerase Chain Reaction-Positives Samples and Positive Cell Cultures, Including 1,941 Severe Acute Respiratory Syndrome Coronavirus 2 Isolates, Clin Infect Dis, doi:10.1093/cid/ciaa1491
Jefferson, Spencer, Brassey, Heneghan, Viral Cultures for Coronavirus Disease 2019 Infectivity Assessment: A Systematic Review, Clin Infect Dis, doi:10.1093/cid/ciaa1764
Magleby, Westblade, Trzebucki, Simon, Rajan et al., Impact of Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load on Risk of Intubation and Mortality Among Hospitalized Patients With Coronavirus Disease, Clin Infect Dis, doi:10.1093/cid/ciaa851
Matson, Yinda, Seifert, Bushmaker, Fischer et al., Effect of Environmental Conditions on SARS-CoV-2 Stability in Human Nasal Mucus and Sputum. Emerg Infect Dis, doi:10.3201/eid2609.202267
Orcina, Santos, Oral manifestation COVID-19 and the rapid resolution of symptoms post-phtalox treatment: A case series, Int. J. Odontostomat, doi:10.4067/S0718-381X2021000100067
Rao, Manissero, Steele, Pareja, A Systematic Review of the Clinical Utility of Cycle Threshold Values in the Context of COVID-19, doi:10.1007/s40121-020-00324-3
Santos, Da Fonseca Orcina, Reia, Ribeiro, Grotto et al., Virucidal Activity of the Antiseptic Mouthwash and Dental Gel Containing Anionic Phthalocyanine Derivative: In vitro Study, Clin Cosmet Investig Dent, doi:10.2147/CCIDE.S315419
Stathis, Victoria, Loomis, Nguyen, Eggers et al., Review of the use of nasal and oral antiseptics during a global pandemic, Future Microbiol, doi:10.2217/fmb-2020-0286
DOI record:
{
"DOI": "10.3205/000321",
"URL": "https://www.egms.de/en/journals/gms/2023-21/000321.shtml",
"abstract": "Background: In individuals with coronavirus disease (COVID-19), the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load (VL) plays an important role in infectivity. Objectives: This study aimed to evaluate the reduction in the VL and infectivity induced by phthalocyanine mouthwash and nasal spray in patients with COVID-19. Methods: Patients with mild COVID-19 were recruited to participate in a triple-blinded randomized controlled trial. Participants were assigned to one of three groups: Group 1, non-active mouthwash and saline nasal spray (SNS); Group 2, phthalocyanine mouthwash and SNS; and Group 3 phthalocyanine mouthwash and phthalocyanine nasal spray. VL was assessed in nasopharyngeal and oropharyngeal swabs collected at the time of clinical diagnosis at baseline as well as 24 and 72 hours after starting the rinsing protocols. Findings: Forty-six participants were included in the analysis: 15, 16, and 15 in Groups 1, 2, and 3, respectively. After 72 hours, the reduction in VL was significantly higher in Group 3 (mean cycle threshold (Ct) decrease: 11.21) than in Group 1 (mean Ct decrease: 5.53). Additionally, only the mean VL in Group 3 was reduced to a non-contagious level after 72 hours. Main conclusions: Use of phthalocyanine mouthwash and nasal spray is effective at reducing SARS-CoV-2 infectivity.",
"author": [
{
"family": "Colado Simão",
"given": "Andréa Name"
},
{
"family": "Perugini Stadtlober",
"given": "Nicole"
},
{
"family": "Stinghen Garcia Lonni",
"given": "Audrey Alesandra"
},
{
"family": "Venâncio",
"given": "Luiza Mara"
},
{
"family": "Lerner Trigo",
"given": "Guilherme"
},
{
"family": "de Souza Cassela",
"given": "Pedro Luis Candido"
},
{
"family": "Mastellini Sanches Silva",
"given": "Thais"
},
{
"family": "De Fátima Oliveira Hirth Ruiz",
"given": "Maria"
},
{
"family": "Batisti Lozovoy",
"given": "Marcell Alysson"
},
{
"family": "Tano",
"given": "Zuleica Naomi"
},
{
"family": "da Fonseca Orcina",
"given": "Bernardo"
},
{
"family": "Vieira Vilhena",
"given": "Fabiano"
},
{
"family": "da Silva Santos",
"given": "Paulo Sérgio"
}
],
"categories": [
"COVID-19",
"SARS-CoV-2 infection",
"infectivity",
"phthalocyanine",
"mouthwashes",
"COVID-19",
"SARS-CoV-2-Infektion",
"Infektiosität",
"Phthalocyanin",
"Mundspülungen",
"Medicine and health"
],
"copyright": "Creative Commons Attribution 4.0 International",
"id": "https://doi.org/10.3205/000321",
"issued": {
"date-parts": [
[
2023,
6,
23
]
]
},
"language": "en",
"publisher": "German Medical Science GMS Publishing House",
"title": "Effect of phthalocyanine oral and nasal antiseptic solutions on the infectivity of SARS-CoV-2 in patients with COVID-19: a randomized controlled trial",
"type": "article-journal"
}