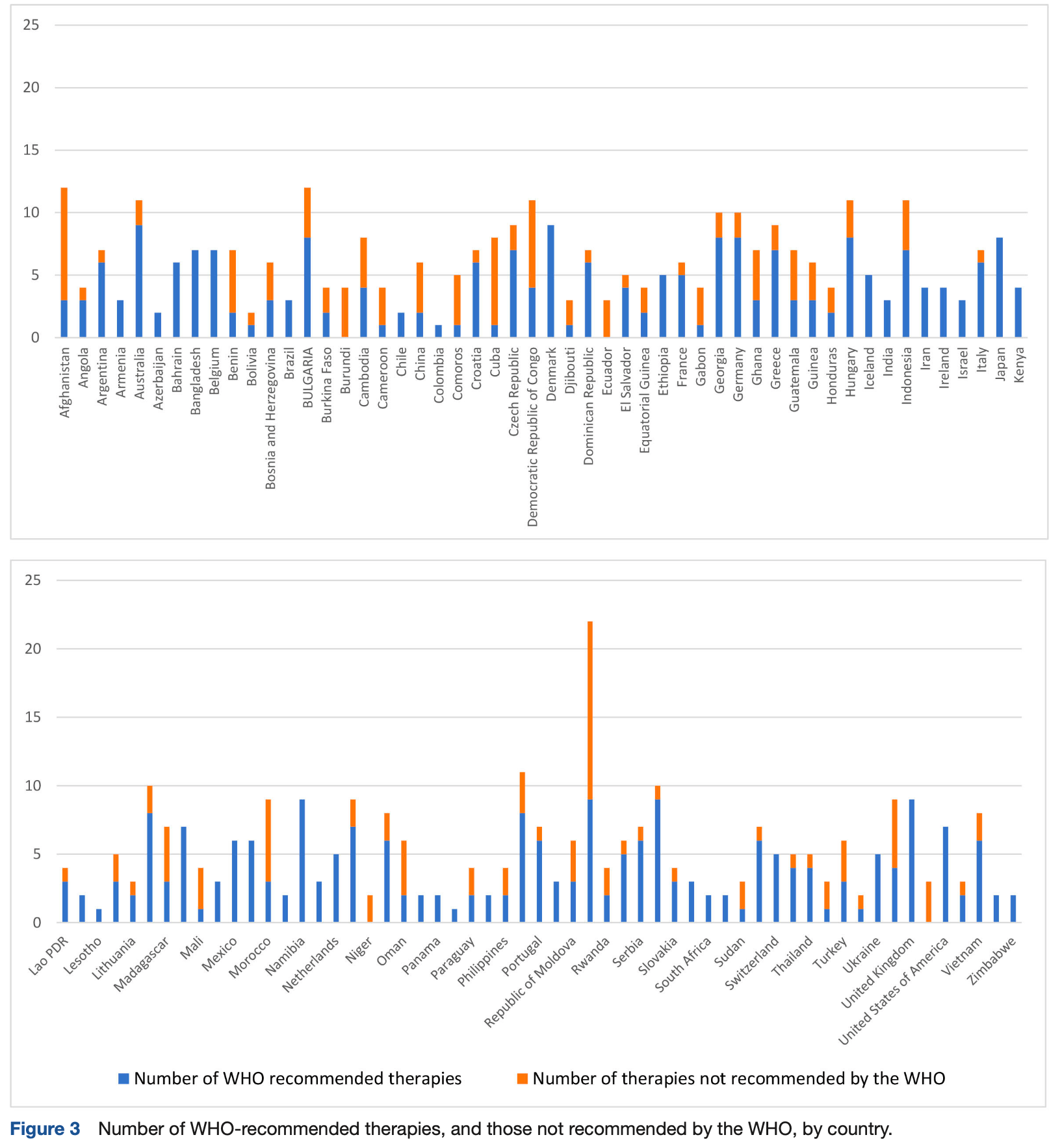
Comparison of WHO versus national COVID-19 therapeutic guidelines across the world: not exactly a perfect match
et al., BMJ Global Health, doi:10.1136/bmjgh-2023-014188, Apr 2024
Comparison of COVID-19 treatment guidelines from 109 countries with WHO guidelines, showing very high variation between national guidelines and frequent recommendations inconsistent with WHO claims. Almost all countries did not follow the WHO guidelines. 93% of national guidelines recommended at least one treatment not recommended by WHO. Authors note that the WHO guidelines were not consistent, and in some cases were confusing or contradictory.
We note that the WHO guidelines did not match the evidence in many cases - many studies were ignored, guidelines were often not updated for months, the results of high conflict of interest studies were inappropriately generalized to more appropriate treatment timing and regimens, and the recommendations exhibited a strong bias toward more profitable decisions.
Cokljat et al., 22 Apr 2024, peer-reviewed, 12 authors, study period September 2022 - December 2022.
Contact: philippe.guerin@iddo.org, nickwdt@tropmedres.a.
Comparison of WHO versus national COVID-19 therapeutic guidelines across the world: not exactly a perfect match
BMJ Global Health, doi:10.1136/bmjgh-2023-014188
Background The COVID-19 pandemic affected all WHO member states. We compared and contrasted the COVID-19 treatment guidelines of each member state with the WHO COVID-19 therapeutic guidelines. Methods Ministries of Health or accessed National Infectious Disease websites and other relevant bodies and experts were contacted to obtain national guidelines (NGs) for COVID-19 treatment. NGs were included only if they delineated specific pharmacological treatments for COVID-19, which were stratified by disease severity. We conducted a retrospective review using the adapted Reporting Checklist for Public Versions of Guidelines (RIGHT-PVG) survey checklist and a derived comparative metric based on the WHO guidelines was performed. Results COVID-19 therapeutics NGs could be obtained from 109 of the 194 WHO member states. There was considerable variation in guidelines and in disease severity stratifications. Therapeutic recommendations in many NGs differed substantially from the WHO guidelines. Overall in late 2022, 93% of NGs were recommending at least one treatment which had proved to be ineffective in large randomised trials, and was not recommended by WHO. Corticosteroids were not recommended in severe disease in nearly 10% of NGs despite overwhelming evidence of their benefit. NGs from countries with low-resource settings showed the greatest divergence when stratified by gross domestic product per year, Human Development Index and the Global Health Security Index. Discussion Our study is limited to NGs that were readily accessible, and it does not reflect the availability of recommended medicines in the field. Three years after the start of the SARS-CoV-2 pandemic, available COVID-19 NGs vary substantially in their therapeutic recommendations, often differ from the WHO guidelines, and commonly recommend ineffective, unaffordable or unavailable medicines.
BMJ Global Health 4 Institute of Global Health, Faculty of Medicine, University of Geneva, Geneve, Switzerland 5 Laboratorio de Estadistica Aplicada a Ciencias de la Salud (LEACS), Facultad de Medicina, Universidad de Buenos Aires, Buenos Aires, Argentina 6 Instituto de Investigacion, Swiss Medical Group, Buenos Aires, Argentina 7 Hospital Italiano de Buenos Aires, Buenos Aires, Argentina Twitter Cintia Valeria Cruz @MedicaMigrante Contributors CVC and MC are both first coauthors of this paper and responsible for the overall content as the guarantors. NJW, PJG, CVC and MC conceived the project. NJW, PG, JT, CVC and MC designed and implemented the study. CVC, MC and JAW conducted the statistical analysis. CVC led all aspects of project management and MC led all aspects of data extraction training and overseeing. CVC, MC, VIC, KP, CC, SMI, MR-P and AR were involved in acquiring the national guidelines and extracting the data. CVC and MC led the project and and wrote the first and final draft of the manuscript. VIC, KP, CC, SMI, AR, JAW, JT, NJW and PJG read and critically revised the manuscript. All authors read and approved the final manuscript. Competing interests None declared. Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research. Patient consent for publication Not applicable. Provenance and peer review Not commissioned; externally peer reviewed. Data availability..
References
Ader, Bouscambert-Duchamp, Hites, Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DISCOVERY): a phase 3, randomised, controlled, open-label trial, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00485-0
Burki, COVID-19 in Latin America, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30303-0
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): preliminary analysis from the United Kingdom randomised, controlled open-label, platform adaptive trial [Lancet, SSRN Journal, doi:10.2139/ssrn.4237902
Echeverría-Esnal, Martin-Ontiyuelo, Navarrete-Rouco, Azithromycin in the treatment of COVID-19: a review, Expert Rev Anti Infect Ther, doi:10.1080/14787210.2020.1813024
El, Spanish, None, AMR
Harris, Taylor, Minor, The Redcap consortium: building an international community of software platform partners, J Biomed Inform, doi:10.1016/j.jbi.2019.103208
Heled, Rutschman, Vertinsky, The problem with relying on profit-driven models to produce pandemic drugs, J Law Biosci, doi:10.1093/jlb/lsaa060
Horby, RECOVERY collaborative group
La, PANDEMIE A CORONAVIRUS COnot mentioned Denmark Danish, O)
Lao, People's Dem Lao language (O) 7, WPR
Li, Hilgenfeld, Whitley, Therapeutic strategies for COVID-19: progress and lessons learned, Nat Rev Drug Discov, doi:10.1038/s41573-023-00672-y
Mclean, Rashan, Tran, The fragmented COVID-19 Therapeutics research landscape: a living systematic review of clinical trial registrations evaluating priority pharmacological interventions, Wellcome Open Res, doi:10.12688/wellcomeopenres.17284.1
Msf, The global COVID-19 treatment divide, doi:10.1016/S0140-6736(22)00372-5
Rome, Avorn, Drug evaluation during the COVID-19 pandemic, N Engl J Med, doi:10.1056/NEJMp2009457
Schilling, Callery, Chandna, The WHO guideline on drugs to prevent COVID-19: small numbers-big conclusions, Wellcome Open Res, doi:10.12688/wellcomeopenres.16741.2
Wang, Chen, Akl, The reporting checklist for public versions of guidelines: RIGHT-PVG, Implement Sci, doi:10.1186/s13012-020-01066-z
White, Strub-Wourgaft, Faiz, Guidelines should not pool evidence from uncomplicated and severe COVID-19, Lancet, doi:10.1016/S0140-6736(21)00469-4
Who, WHO COVID-19 solidarity Therapeutics trial
DOI record:
{
"DOI": "10.1136/bmjgh-2023-014188",
"ISSN": [
"2059-7908"
],
"URL": "http://dx.doi.org/10.1136/bmjgh-2023-014188",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>The COVID-19 pandemic affected all WHO member states. We compared and contrasted the COVID-19 treatment guidelines of each member state with the WHO COVID-19 therapeutic guidelines.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Ministries of Health or accessed National Infectious Disease websites and other relevant bodies and experts were contacted to obtain national guidelines (NGs) for COVID-19 treatment. NGs were included only if they delineated specific pharmacological treatments for COVID-19, which were stratified by disease severity. We conducted a retrospective review using the adapted Reporting Checklist for Public Versions of Guidelines (RIGHT-PVG) survey checklist and a derived comparative metric based on the WHO guidelines was performed.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>COVID-19 therapeutics NGs could be obtained from 109 of the 194 WHO member states. There was considerable variation in guidelines and in disease severity stratifications. Therapeutic recommendations in many NGs differed substantially from the WHO guidelines. Overall in late 2022, 93% of NGs were recommending at least one treatment which had proved to be ineffective in large randomised trials, and was not recommended by WHO. Corticosteroids were not recommended in severe disease in nearly 10% of NGs despite overwhelming evidence of their benefit. NGs from countries with low-resource settings showed the greatest divergence when stratified by gross domestic product per year, Human Development Index and the Global Health Security Index.</jats:p></jats:sec><jats:sec><jats:title>Discussion</jats:title><jats:p>Our study is limited to NGs that were readily accessible, and it does not reflect the availability of recommended medicines in the field. Three years after the start of the SARS-CoV-2 pandemic, available COVID-19 NGs vary substantially in their therapeutic recommendations, often differ from the WHO guidelines, and commonly recommend ineffective, unaffordable or unavailable medicines.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2024,
2,
26
]
]
},
"alternative-id": [
"10.1136/bmjgh-2023-014188"
],
"author": [
{
"affiliation": [],
"family": "Cokljat",
"given": "Mia",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-8393-8536",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cruz",
"given": "Cintia Valeria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carrara",
"given": "Verena Ilona",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Puttaraska",
"given": "Kanoktip",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Capriglioni",
"given": "Camila",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Insaurralde",
"given": "Sabrina Marcela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rousseau-Portalis",
"given": "Maximo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roldan",
"given": "Agustina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5524-0325",
"affiliation": [],
"authenticated-orcid": false,
"family": "Watson",
"given": "James A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tarning",
"given": "Joel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "White",
"given": "Nicholas J",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6008-2963",
"affiliation": [],
"authenticated-orcid": false,
"family": "Guerin",
"given": "Philippe J",
"sequence": "additional"
}
],
"container-title": "BMJ Global Health",
"container-title-short": "BMJ Glob Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2024,
4,
23
]
],
"date-time": "2024-04-23T00:00:12Z",
"timestamp": 1713830412000
},
"deposited": {
"date-parts": [
[
2024,
4,
23
]
],
"date-time": "2024-04-23T00:00:28Z",
"timestamp": 1713830428000
},
"indexed": {
"date-parts": [
[
2024,
4,
25
]
],
"date-time": "2024-04-25T11:57:38Z",
"timestamp": 1714046258195
},
"is-referenced-by-count": 1,
"issue": "4",
"issued": {
"date-parts": [
[
2024,
4
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2024,
4,
22
]
]
},
"published-print": {
"date-parts": [
[
2024,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T00:00:00Z",
"timestamp": 1711670400000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmjgh-2023-014188",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e014188",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2024,
4
]
]
},
"published-online": {
"date-parts": [
[
2024,
4,
22
]
]
},
"published-print": {
"date-parts": [
[
2024,
4
]
]
},
"publisher": "BMJ",
"reference": [
{
"key": "2024042217000524000_9.4.e014188.1",
"unstructured": "Schellekens P . A life lost is a life lost, Available: https://pandem-ic.com/"
},
{
"key": "2024042217000524000_9.4.e014188.2",
"unstructured": "WHO . WHO Coronavirus (COVID-19) dashboard. situation by region, country, territory & area. 2023. Available: https://covid19.who.int/table"
},
{
"DOI": "10.12688/wellcomeopenres.17284.1",
"article-title": "The fragmented COVID-19 Therapeutics research landscape: a living systematic review of clinical trial registrations evaluating priority pharmacological interventions",
"author": "McLean",
"doi-asserted-by": "crossref",
"first-page": "24",
"journal-title": "Wellcome Open Res",
"key": "2024042217000524000_9.4.e014188.3",
"volume": "7",
"year": "2022"
},
{
"key": "2024042217000524000_9.4.e014188.4",
"unstructured": "Infectious Diseases Data Observatory . A living systematic review of registered COVID-19 trials. 2023. Available: https://www.iddo.org/covid-19/live-systematic-review-trials"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "2024042217000524000_9.4.e014188.5"
},
{
"key": "2024042217000524000_9.4.e014188.6",
"unstructured": "Horby PMM . RECOVERY collaborative group. 2023. Available: https://www.recoverytrial.net"
},
{
"key": "2024042217000524000_9.4.e014188.7",
"unstructured": "WHO . WHO COVID-19 solidarity Therapeutics trial. 2020. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments"
},
{
"key": "2024042217000524000_9.4.e014188.8",
"unstructured": "REMAP . Publications and results — REMAP-CAP trial. 2023. Available: https://www.remapcap.org/covid19publications"
},
{
"DOI": "10.1056/NEJMp2009457",
"doi-asserted-by": "publisher",
"key": "2024042217000524000_9.4.e014188.9"
},
{
"DOI": "10.1016/S1473-3099(20)30303-0",
"doi-asserted-by": "publisher",
"key": "2024042217000524000_9.4.e014188.10"
},
{
"key": "2024042217000524000_9.4.e014188.11",
"unstructured": "World Health Organization . Therapeutics and COVID-19: living guideline. 2022."
},
{
"DOI": "10.1016/S0140-6736(21)00469-4",
"article-title": "Guidelines should not pool evidence from uncomplicated and severe COVID-19",
"author": "White",
"doi-asserted-by": "crossref",
"first-page": "1262",
"journal-title": "Lancet",
"key": "2024042217000524000_9.4.e014188.12",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1080/14787210.2020.1813024",
"doi-asserted-by": "publisher",
"key": "2024042217000524000_9.4.e014188.13"
},
{
"DOI": "10.1016/j.jbi.2019.103208",
"article-title": "The Redcap consortium: building an international community of software platform partners",
"author": "Harris",
"doi-asserted-by": "crossref",
"journal-title": "J Biomed Inform",
"key": "2024042217000524000_9.4.e014188.14",
"volume": "95",
"year": "2019"
},
{
"DOI": "10.1186/s13012-020-01066-z",
"article-title": "The reporting checklist for public versions of guidelines: RIGHT-PVG",
"author": "Wang",
"doi-asserted-by": "crossref",
"journal-title": "Implement Sci",
"key": "2024042217000524000_9.4.e014188.15",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.12688/wellcomeopenres.16741.1",
"article-title": "The WHO guideline on drugs to prevent COVID-19: small numbers- big conclusions",
"author": "Schilling",
"doi-asserted-by": "crossref",
"journal-title": "Wellcome Open Res",
"key": "2024042217000524000_9.4.e014188.16",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1038/s41573-023-00672-y",
"article-title": "Therapeutic strategies for COVID-19: progress and lessons learned",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "449",
"journal-title": "Nat Rev Drug Discov",
"key": "2024042217000524000_9.4.e014188.17",
"volume": "22",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(21)00485-0",
"article-title": "Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DISCOVERY): a phase 3, randomised, controlled, open-label trial",
"author": "Ader",
"doi-asserted-by": "crossref",
"first-page": "209",
"journal-title": "Lancet Infect Dis",
"key": "2024042217000524000_9.4.e014188.18",
"volume": "22",
"year": "2022"
},
{
"key": "2024042217000524000_9.4.e014188.19",
"unstructured": "WHO drugs to prevent COVID-19: A WHO living guideline. 2021."
},
{
"article-title": "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): preliminary analysis from the United Kingdom randomised, controlled open-label, platform adaptive trial",
"author": "Butler",
"first-page": "281",
"journal-title": "SSRN Journal",
"key": "2024042217000524000_9.4.e014188.20",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1093/jlb/lsaa060",
"article-title": "The problem with relying on profit-driven models to produce pandemic drugs",
"author": "Heled",
"doi-asserted-by": "crossref",
"journal-title": "J Law Biosci",
"key": "2024042217000524000_9.4.e014188.21",
"volume": "7",
"year": "2020"
},
{
"key": "2024042217000524000_9.4.e014188.22",
"unstructured": "Agence France-Presse (AFP) . US buys up almost entire world supply of coronavirus drug remdesivir. 2020. Available: https://www.abc.net.au/news/2020-07-02/us-criticised-hoarding-coronavirus-covid19-drug-remdesivir/12414154"
},
{
"key": "2024042217000524000_9.4.e014188.23",
"unstructured": "MSF . New MSF report: high-income countries must stop Hoarding 870 million excess COVID-19 vaccines doses and Redistribute them to save lives - doctors without borders / Médecins Sans Frontières. 2021. Available: https://www.doctorswithoutborders.ca/new-msf-report-high-income-countries-must-stop-hoarding-870-million-excess-covid-19-vaccines-doses-and-redistribute-them-to-save-lives"
},
{
"DOI": "10.1016/S0140-6736(22)00372-5",
"article-title": "The global COVID-19 treatment divide",
"author": "Usher",
"doi-asserted-by": "crossref",
"first-page": "779",
"journal-title": "Lancet",
"key": "2024042217000524000_9.4.e014188.24",
"volume": "399",
"year": "2022"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://gh.bmj.com/lookup/doi/10.1136/bmjgh-2023-014188"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Comparison of WHO versus national COVID-19 therapeutic guidelines across the world: not exactly a perfect match",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "9"
}