Oral Azvudine (FNC) Tablets in Patients infected with SARS-CoV-2 Omicron Variant: A Retrospective Cohort Study
et al., medRxiv, doi:10.1101/2023.01.05.23284180, Jan 2023
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 207 COVID-19 patients in China, showing azvudine associated with faster viral clearance, with azvudine-treated patients obtaining a negative PCR test result 1.7 days faster on average compared to supportive care alone after adjusting for age and sex.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
recovery time, 12.5% higher, relative time 1.12, p = 0.94, treatment 66, control 41.
|
|
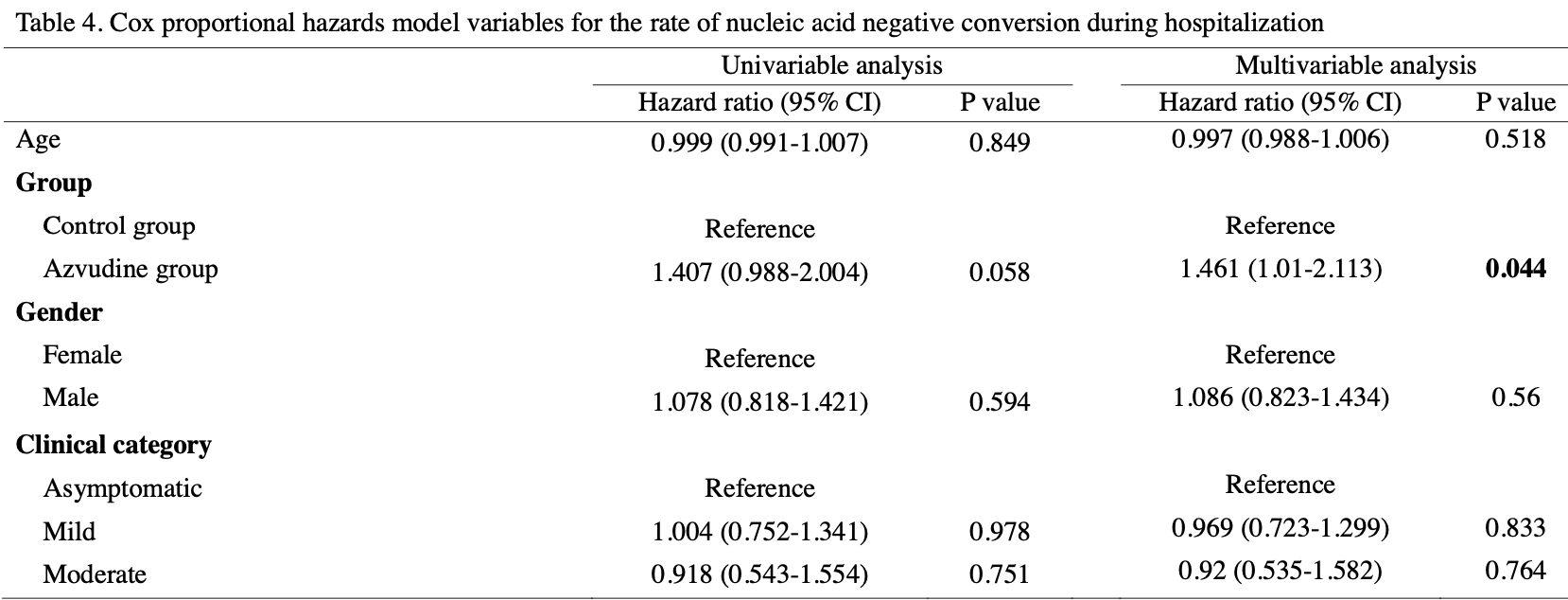
risk of no viral clearance, 31.6% lower, HR 0.68, p = 0.04, treatment 166, control 41, adjusted per study, inverted to make HR<1 favor treatment, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Chen et al., 6 Jan 2023, retrospective, China, preprint, 7 authors, study period August 2022 - October 2022.
Contact: bbmcliwei@126.com, john131212@111.com.
Oral Azvudine (FNC) Tablets in Patients infected with SARS-CoV-2 Omicron Variant: A Retrospective Cohort Study
doi:10.1101/2023.01.05.23284180
Background: There is a lack of data on the efficacy of oral Azvudine in Coronavirus Disease treatment. This study aimed to assess the association between Azvudine treatment and clinical outcomes in a cohort of patients infected with the SARS-CoV-2 Omicron variant. Methods: This is a retrospective study conducted in two mobile cabin hospitals. All consecutive patients with a diagnosis of COVID-19 admitted from August to October 2022 were included in the study. Linear regression models and Cox proportional hazards models were used to assess the association between Azvudine treatment and time to obtain the first negative nucleic acid test results. Results: A total of 207 patients were analyzed, of whom 166 patients (80.2%) received Azvudine treatment after hospitalization, and the rest did not. Linear regression models showed that Azvudine treatment was associated with reduced time to obtain the first negative nucleic acid test results after adjusting for age and gender [mean difference = -1.658; 95% CI: -2.772 to -0.544, P = 0.0039]. The multivariable Cox analysis conforms to the results from the linear regression model (hazard ratio = 1.461; 95% CI: 1.01 to 2.11, P = 0.044).
Conclusion: Azvudine treatment was associated with reduced virus shedding time. Further studies are needed to confirm our findings.
Declarations Acknowledgment: None.
Competing interest: The authors declare no competing interests. Authors' contributions: WL, GQW equally contributed to the conception and design of the research. WMC, HGX, LH, RY and CQP performed the research and analyzed the data. WMC, HGX and LH wrote the manuscript and interpretation of data. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript. Ethics statement: This study was approved by the institutional review board of the First Affiliated Hospital of Bengbu Medical College with a waiver of consent for the the retrospective observational design (No. 2020KY112, 2020KY016). Data are presented as n (%) or median (interquartile range).
References
Arbel, Sagy, Hoshen, Battat, Lavie et al., Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge, N Engl J Med
Brussow, COVID-19: Omicron -the latest, the least virulent, but probably not the last variant of concern of SARS-CoV-2, Microb Biotechnol
Chang, 4'-Modified Nucleosides for Antiviral Drug Discovery: Achievements and Perspectives, Acc Chem Res
Grein, Ohmagari, Shin, Diaz, Asperges et al., Compassionate Use of Remdesivir for Patients with Severe Covid-19, N Engl J Med
Guo, Han, Zhang, He, Yu et al., SARS-CoV-2 Omicron Variant: Epidemiological Features, Biological Characteristics, and Clinical Significance, Front Immunol
Huang, Wu, Zheng, Xie, He et al., Atlas of currently available human neutralizing antibodies against SARS-CoV-2 and escape by Omicron sub-variants BA.1/BA.1, BA.2/BA.3. Immunity
Lin, Wu, Li, Bian, Li et al., Impact of combination preventative interventions on hospitalization and death under the pandemic of SARS-CoV-2 Omicron variant in China, J Med Virol
Mccallum, Czudnochowski, Rosen, Zepeda, Bowen et al., Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement, Science
Ren, Luo, Yu, Song, Liang et al., A Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study, Adv Sci (Weinh)
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Xu, Chen, Tang, Guidelines for the diagnosis and treatment of coronavirus disease 2019 (COVID-19) in China, Glob Health Med
Xu, Li, You, Zhang, Li et al., Effectiveness of inactivated COVID-19 vaccines against mild disease, pneumonia, and severe disease among persons infected with SARS-CoV-2 Omicron variant: Real-world study in Jilin Province, China, Emerg Microbes Infect
Ying-Hao, Yuan-Yuan, Dong, Qiu-Hua, Xue-Ran et al., Clinical characteristics and analysis of risk factors for disease progression of patients with SARS-CoV-2 Omicron variant infection: A retrospective study of 25207 cases in a Fangcang hospital, Front Cell Infect Microbiol
Yu, Chang, Azvudine (FNC): a promising clinical candidate for COVID-19 treatment, Signal Transduct Target Ther
Yu, Chang, The first Chinese oral anti-COVID-19 drug Azvudine launched, Innovation (Camb)
Zhang, Li, Wang, Liu, Lu et al., Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct Target Ther
Zhu, Zhang, Li, Yang, Song, A Novel Coronavirus from Patients with Pneumonia in China, N Engl J Med
DOI record:
{
"DOI": "10.1101/2023.01.05.23284180",
"URL": "http://dx.doi.org/10.1101/2023.01.05.23284180",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>There is a lack of data on the efficacy of oral Azvudine in Coronavirus Disease treatment. This study aimed to assess the association between Azvudine treatment and clinical outcomes in a cohort of patients infected with the SARS-CoV-2 Omicron variant.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>This is a retrospective study conducted in two mobile cabin hospitals. All consecutive patients with a diagnosis of COVID-19 admitted from August to October 2022 were included in the study. Linear regression models and Cox proportional hazards models were used to assess the association between Azvudine treatment and time to obtain the first negative nucleic acid test results.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>A total of 207 patients were analyzed, of whom 166 patients (80.2%) received Azvudine treatment after hospitalization, and the rest did not. Linear regression models showed that Azvudine treatment was associated with reduced time to obtain the first negative nucleic acid test results after adjusting for age and gender [mean difference = −1.658; 95% CI: −2.772 to −0.544, P = 0.0039]. The multivariable Cox analysis conforms to the results from the linear regression model (hazard ratio = 1.461; 95% CI: 1.01 to 2.11, P = 0.044).</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Azvudine treatment was associated with reduced virus shedding time. Further studies are needed to confirm our findings.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2023,
1,
6
]
]
},
"author": [
{
"affiliation": [],
"family": "Chen",
"given": "Wenmei",
"sequence": "first"
},
{
"affiliation": [],
"family": "Xu",
"given": "Honggang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hong",
"given": "Lei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Ran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peng",
"given": "Caiqiu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Guiqiang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Wei",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
1,
6
]
],
"date-time": "2023-01-06T18:15:14Z",
"timestamp": 1673028914000
},
"deposited": {
"date-parts": [
[
2023,
1,
9
]
],
"date-time": "2023-01-09T05:50:19Z",
"timestamp": 1673243419000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2023,
9,
16
]
],
"date-time": "2023-09-16T16:54:30Z",
"timestamp": 1694883270616
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2023,
1,
6
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2023.01.05.23284180",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2023,
1,
6
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2023,
1,
6
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "publisher",
"key": "2023010821500443000_2023.01.05.23284180v1.1"
},
{
"DOI": "10.1038/s41564-020-0695-z",
"article-title": "The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2",
"author": "Coronaviridae Study Group of the International Committee on Taxonomy of V",
"doi-asserted-by": "crossref",
"first-page": "536",
"journal-title": "Nat Microbiol",
"key": "2023010821500443000_2023.01.05.23284180v1.2",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1111/1751-7915.14064",
"doi-asserted-by": "publisher",
"key": "2023010821500443000_2023.01.05.23284180v1.3"
},
{
"DOI": "10.1126/science.abn8652",
"article-title": "Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement",
"doi-asserted-by": "crossref",
"first-page": "864",
"journal-title": "Science",
"key": "2023010821500443000_2023.01.05.23284180v1.4",
"volume": "375",
"year": "2022"
},
{
"DOI": "10.1016/j.immuni.2022.06.005",
"doi-asserted-by": "publisher",
"key": "2023010821500443000_2023.01.05.23284180v1.5"
},
{
"DOI": "10.35772/ghm.2020.01015",
"article-title": "Guidelines for the diagnosis and treatment of coronavirus disease 2019 (COVID-19) in China",
"doi-asserted-by": "crossref",
"first-page": "66",
"journal-title": "Glob Health Med",
"key": "2023010821500443000_2023.01.05.23284180v1.6",
"volume": "2",
"year": "2020"
},
{
"key": "2023010821500443000_2023.01.05.23284180v1.7",
"unstructured": "Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Bethesda (MD) 2021."
},
{
"DOI": "10.1021/acs.accounts.1c00697",
"article-title": "4’-Modified Nucleosides for Antiviral Drug Discovery: Achievements and Perspectives",
"doi-asserted-by": "crossref",
"first-page": "565",
"journal-title": "Acc Chem Res",
"key": "2023010821500443000_2023.01.05.23284180v1.8",
"volume": "55",
"year": "2022"
},
{
"article-title": "The first Chinese oral anti-COVID-19 drug Azvudine launched",
"first-page": "100321",
"journal-title": "Innovation (Camb)",
"key": "2023010821500443000_2023.01.05.23284180v1.9",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1002/advs.202001435",
"article-title": "A Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study",
"doi-asserted-by": "crossref",
"first-page": "e2001435",
"journal-title": "Adv Sci (Weinh)",
"key": "2023010821500443000_2023.01.05.23284180v1.10",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1080/22221751.2022.2149935",
"doi-asserted-by": "crossref",
"key": "2023010821500443000_2023.01.05.23284180v1.11",
"unstructured": "Xu H , Li H , You H , Zhang P , Li N , Jiang N , et al. Effectiveness of inactivated COVID-19 vaccines against mild disease, pneumonia, and severe disease among persons infected with SARS-CoV-2 Omicron variant: Real-world study in Jilin Province, China. Emerg Microbes Infect 1–30 (2022)."
},
{
"DOI": "10.1002/jmv.28335",
"doi-asserted-by": "crossref",
"key": "2023010821500443000_2023.01.05.23284180v1.12",
"unstructured": "Lin YF , Wu X , Li Y , Bian J , Li K , Jiang Y , et al. Impact of combination preventative interventions on hospitalization and death under the pandemic of SARS-CoV-2 Omicron variant in China. J Med Virol (2022)."
},
{
"DOI": "10.3389/fcimb.2022.1009894",
"article-title": "Clinical characteristics and analysis of risk factors for disease progression of patients with SARS-CoV-2 Omicron variant infection: A retrospective study of 25207 cases in a Fangcang hospital",
"doi-asserted-by": "crossref",
"first-page": "1009894",
"journal-title": "Front Cell Infect Microbiol",
"key": "2023010821500443000_2023.01.05.23284180v1.13",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2022.877101",
"doi-asserted-by": "publisher",
"key": "2023010821500443000_2023.01.05.23284180v1.14"
},
{
"DOI": "10.1038/s41392-020-00351-z",
"article-title": "Azvudine (FNC): a promising clinical candidate for COVID-19 treatment",
"doi-asserted-by": "crossref",
"first-page": "236",
"journal-title": "Signal Transduct Target Ther",
"key": "2023010821500443000_2023.01.05.23284180v1.15",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"article-title": "Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients",
"doi-asserted-by": "crossref",
"first-page": "414",
"journal-title": "Signal Transduct Target Ther",
"key": "2023010821500443000_2023.01.05.23284180v1.16",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "2023010821500443000_2023.01.05.23284180v1.17"
},
{
"DOI": "10.1056/NE-JMoa2007016",
"doi-asserted-by": "publisher",
"key": "2023010821500443000_2023.01.05.23284180v1.18"
},
{
"DOI": "10.1056/NEJMoa2204919",
"doi-asserted-by": "publisher",
"key": "2023010821500443000_2023.01.05.23284180v1.19"
}
],
"reference-count": 19,
"references-count": 19,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2023.01.05.23284180"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Oral Azvudine (FNC) Tablets in Patients infected with SARS-CoV-2 Omicron Variant: A Retrospective Cohort Study",
"type": "posted-content"
}
