Antiandrogens for the treatment of COVID‐19 patients: A meta‐analysis of randomized controlled trials
et al., Journal of Medical Virology, doi:10.1002/jmv.28740, Apr 2023
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
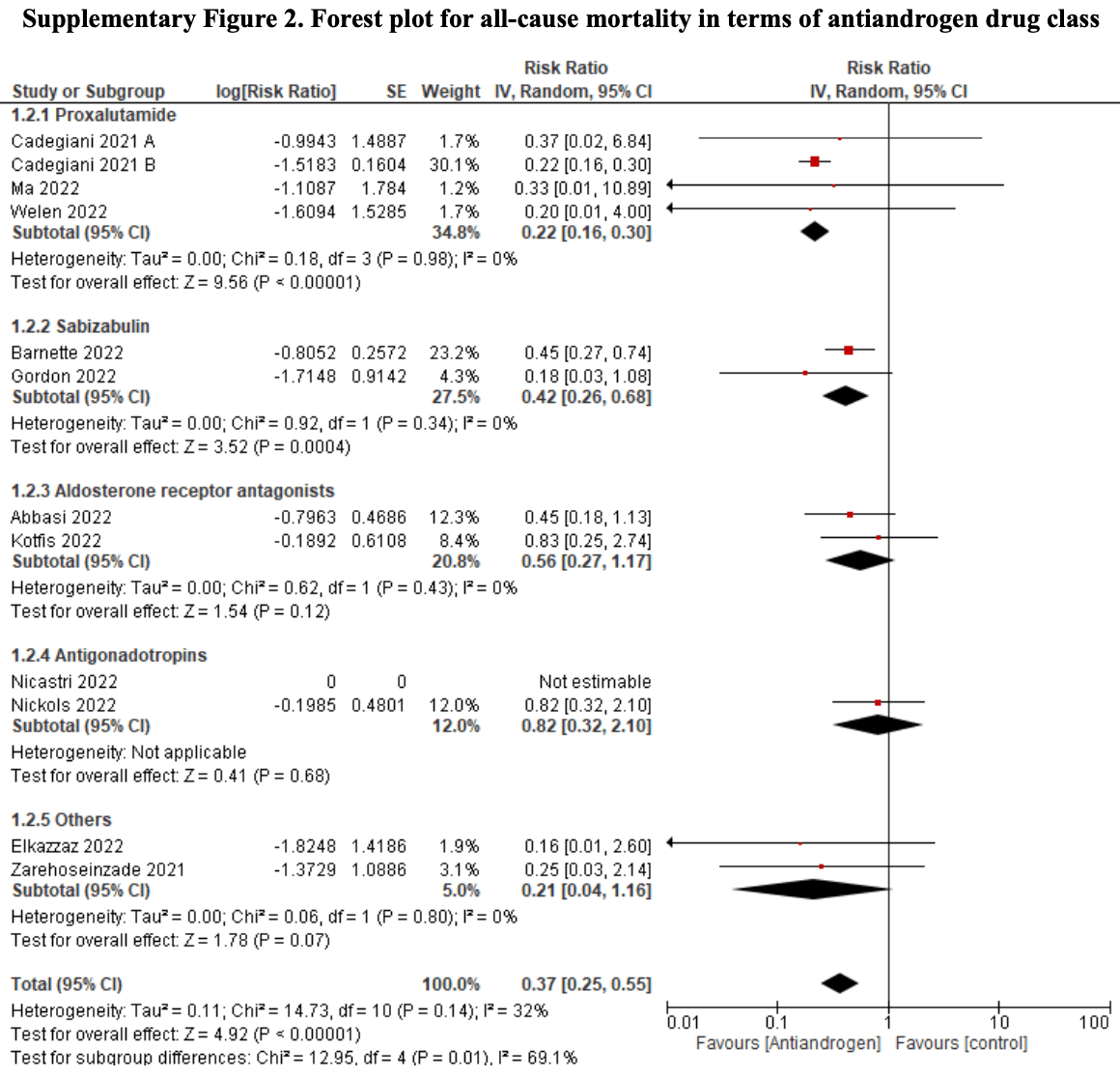
Meta analysis of 14 antiandrogen RCTs for COVID-19, showing significantly lower mortality with treatment.
2 meta-analyses show significant improvements with antiandrogens for mortality1,2,
hospitalization2,
recovery2, and
progression1.
Currently there are 49 antiandrogens for COVID-19 studies, showing 37% lower mortality [21‑50%], 47% lower ventilation [23‑64%], 36% lower ICU admission [5‑57%], 32% lower hospitalization [11‑48%], and 8% fewer cases [1‑14%].
|
risk of death, 63.0% lower, RR 0.37, p < 0.001.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Cheema et al., 18 Apr 2023, peer-reviewed, 9 authors.
DOI record:
{
"DOI": "10.1002/jmv.28740",
"ISSN": [
"0146-6615",
"1096-9071"
],
"URL": "http://dx.doi.org/10.1002/jmv.28740",
"alternative-id": [
"10.1002/jmv.28740"
],
"author": [
{
"affiliation": [
{
"name": "Department of Medicine, Division of Infectious Diseases King Edward Medical University Lahore Pakistan"
}
],
"family": "Cheema",
"given": "Huzaifa Ahmad",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-3795-8998",
"affiliation": [
{
"name": "Department of Medicine, Division of Infectious Diseases King Edward Medical University Lahore Pakistan"
}
],
"authenticated-orcid": false,
"family": "Rehman",
"given": "Aqeeb Ur",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine Kafr El‐Shaikh University Kafr El‐Shaikh Egypt"
}
],
"family": "Elrashedy",
"given": "Asmaa Ahmed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Division of Infectious Diseases King Edward Medical University Lahore Pakistan"
}
],
"family": "Mohsin",
"given": "Aleenah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Division of Infectious Diseases King Edward Medical University Lahore Pakistan"
}
],
"family": "Shahid",
"given": "Abia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6021-2342",
"affiliation": [
{
"name": "Department of Medicine, Division of Infectious Diseases King Edward Medical University Lahore Pakistan"
}
],
"authenticated-orcid": false,
"family": "Ehsan",
"given": "Muhammad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Division of Infectious Diseases King Edward Medical University Lahore Pakistan"
}
],
"family": "Ayyan",
"given": "Muhammad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine Limerick University Hospital Limerick Ireland"
}
],
"family": "Ismail",
"given": "Heba",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Galway University Hospital Galway Ireland"
}
],
"family": "Almas",
"given": "Talal",
"sequence": "additional"
}
],
"container-title": "Journal of Medical Virology",
"container-title-short": "Journal of Medical Virology",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
4,
18
]
],
"date-time": "2023-04-18T12:41:07Z",
"timestamp": 1681821667000
},
"deposited": {
"date-parts": [
[
2023,
4,
18
]
],
"date-time": "2023-04-18T12:41:21Z",
"timestamp": 1681821681000
},
"indexed": {
"date-parts": [
[
2023,
4,
19
]
],
"date-time": "2023-04-19T05:36:31Z",
"timestamp": 1681882591366
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2023,
4
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2023,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 17,
"start": {
"date-parts": [
[
2023,
4,
18
]
],
"date-time": "2023-04-18T00:00:00Z",
"timestamp": 1681776000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.28740",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2023,
4
]
]
},
"published-online": {
"date-parts": [
[
2023,
4,
18
]
]
},
"published-print": {
"date-parts": [
[
2023,
4
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.37201/req/158.2021",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_2_1"
},
{
"DOI": "10.1016/j.ejim.2022.05.024",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_3_1"
},
{
"DOI": "10.1002/jmv.28471",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_4_1"
},
{
"DOI": "10.1002/ptr.7724",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_5_1"
},
{
"DOI": "10.1002/rmv.2427",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_6_1"
},
{
"DOI": "10.1016/j.jinf.2022.11.022",
"article-title": "No evidence of clinical efficacy of famotidine for the treatment of COVID‐19 in a systematic review and meta‐analysis",
"author": "Cheema HA",
"doi-asserted-by": "crossref",
"first-page": "154",
"journal-title": "J Infect",
"key": "e_1_2_8_7_1",
"volume": "86",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2022.10.012",
"article-title": "Efficacy and safety of fluvoxamine for the treatment of COVID‐19 patients: a systematic review and meta‐analysis",
"author": "Cheema HA",
"doi-asserted-by": "crossref",
"first-page": "702",
"issue": "6",
"journal-title": "J Infect",
"key": "e_1_2_8_8_1",
"volume": "85",
"year": "2022"
},
{
"DOI": "10.1136/jclinpath-2020-206987",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_9_1"
},
{
"DOI": "10.1136/bmj.n71",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_10_1"
},
{
"DOI": "10.1136/bmj.l4898",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_11_1"
},
{
"DOI": "10.1210/jendso/bvac017",
"article-title": "A randomized trial of sitagliptin and spironolactone with combination therapy in hospitalized adults with COVID‐19",
"author": "Abbasi F",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "4",
"journal-title": "J Endocr Soc",
"key": "e_1_2_8_12_1",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1056/EVIDoa2200145",
"article-title": "Oral sabizabulin for high‐risk, hospitalized adults with Covid‐19: interim analysis",
"author": "Barnette KG",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "NEJM Evid",
"key": "e_1_2_8_13_1",
"volume": "1",
"year": "2022"
},
{
"DOI": "10.1016/j.eururo.2021.12.013",
"article-title": "A phase 2 trial of the effect of antiandrogen therapy on COVID‐19 outcome: no evidence of benefit, supported by epidemiology and in vitro data",
"author": "Welén K",
"doi-asserted-by": "crossref",
"first-page": "285",
"issue": "3",
"journal-title": "Eur Urol",
"key": "e_1_2_8_14_1",
"volume": "81",
"year": "2022"
},
{
"article-title": "Finasteride in hospitalized adult males with COVID‐19: a risk factor for severity of the disease or an adjunct treatment: a randomized controlled clinical trial",
"author": "Zarehoseinzade E",
"issue": "1",
"journal-title": "Med J Islam Repub Iran",
"key": "e_1_2_8_15_1",
"volume": "35",
"year": "2021"
},
{
"key": "e_1_2_8_16_1",
"unstructured": "NCT04870606. Proxalutamide (GT0918) treatment for outpatients with mild or moderate COVID‐19 illnessClinicalTrials.gov.2022. Accessed March 8 2023.https://www.prnewswire.com/news-releases/kintor-pharmas-proxalutamide-demonstrated-reduction-in-hospitalizationmortality-for-patients-with-mild-to-moderate-covid-19-in-phase-iii-mrct-study-301518525.html"
},
{
"key": "e_1_2_8_17_1",
"unstructured": "Veru Inc. COVID‐19 Treatment of Severe Acute Respiratory Syndrome With Veru‐111.ClinicalTrials.gov.2021. Accessed March 8 2023.https://clinicaltrials.gov/ct2/show/results/NCT04388826"
},
{
"article-title": "Proxalutamide (GT0918) reduces the rate of hospitalization in mild‐to‐moderate COVID‐19 female patients: a randomized double‐blinded placebo‐controlled two‐arm parallel trial",
"author": "Adsuara Cadegiani F",
"journal-title": "medRxiv",
"key": "e_1_2_8_18_1",
"year": "2021"
},
{
"article-title": "Final results of a randomized, placebo‐controlled, two‐arm, parallel clinical trial of proxalutamide for hospitalized COVID‐19 patients: a multiregional, joint analysis of the proxa‐rescue AndroCoV trial",
"author": "Cadegiani FA",
"issue": "12",
"journal-title": "Cureus",
"key": "e_1_2_8_19_1",
"volume": "13",
"year": "2021"
},
{
"article-title": "Early antiandrogen therapy with dutasteride reduces viral shedding, inflammatory responses, and time‐to‐remission in males with COVID‐19: a randomized, double‐blind, placebo‐controlled interventional trial (EAT‐DUTA AndroCoV trial–biochemical)",
"author": "Cadegiani FA",
"issue": "2",
"journal-title": "Cureus",
"key": "e_1_2_8_20_1",
"volume": "13",
"year": "2021"
},
{
"article-title": "13 cis retinoic acid improved the outcomes of COVID‐19 patients. A randomized clinical trial",
"author": "Elkazzaz M",
"journal-title": "medRxiv",
"key": "e_1_2_8_21_1",
"year": "2022"
},
{
"article-title": "Mineralocorticoid receptor antagonist (potassium canrenoate) does not influence outcome in the treatment of COVID‐19‐associated pneumonia and Fibrosis—a randomized placebo controlled clinical trial",
"author": "Kotfis K",
"issue": "2",
"journal-title": "Pharm",
"key": "e_1_2_8_22_1",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2022.101450",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_23_1"
},
{
"DOI": "10.1001/jamanetworkopen.2022.7852",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_24_1"
},
{
"article-title": "Phase 2 randomised placebo‐controlled trial of spironolactone and dexamethasone versus dexamethasone in COVID‐19 hospitalised patients in Delhi",
"author": "Wadhwa B",
"journal-title": "medRxiv",
"key": "e_1_2_8_25_1",
"year": "2022"
},
{
"DOI": "10.1530/ERC-20-0165",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_26_1"
},
{
"DOI": "10.1007/s00345-021-03810-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_27_1"
},
{
"article-title": "Effects of androgen deprivation therapy on COVID‐19 in patients with prostate cancer: a systematic review and meta‐analysis",
"author": "Karimi A",
"first-page": "577",
"journal-title": "Urol J",
"key": "e_1_2_8_28_1",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2022.03.020",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_29_1"
},
{
"DOI": "10.1016/j.coi.2009.05.023",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_30_1"
},
{
"DOI": "10.1093/cid/ciaa1022",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_31_1"
},
{
"DOI": "10.1016/bs.apcsb.2019.01.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_32_1"
},
{
"DOI": "10.1093/biolre/ioy043",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_33_1"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/jmv.28740"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Antiandrogens for the treatment of COVID‐19 patients: A meta‐analysis of randomized controlled trials",
"type": "journal-article",
"volume": "95"
}
