Effect of vitamin D3 supplementation on cellular immunity and inflammatory markers in COVID-19 patients admitted to the ICU
et al., Scientific Reports, doi:10.1038/s41598-022-22045-y, COVID-VIT, NCT05092698, Nov 2022
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
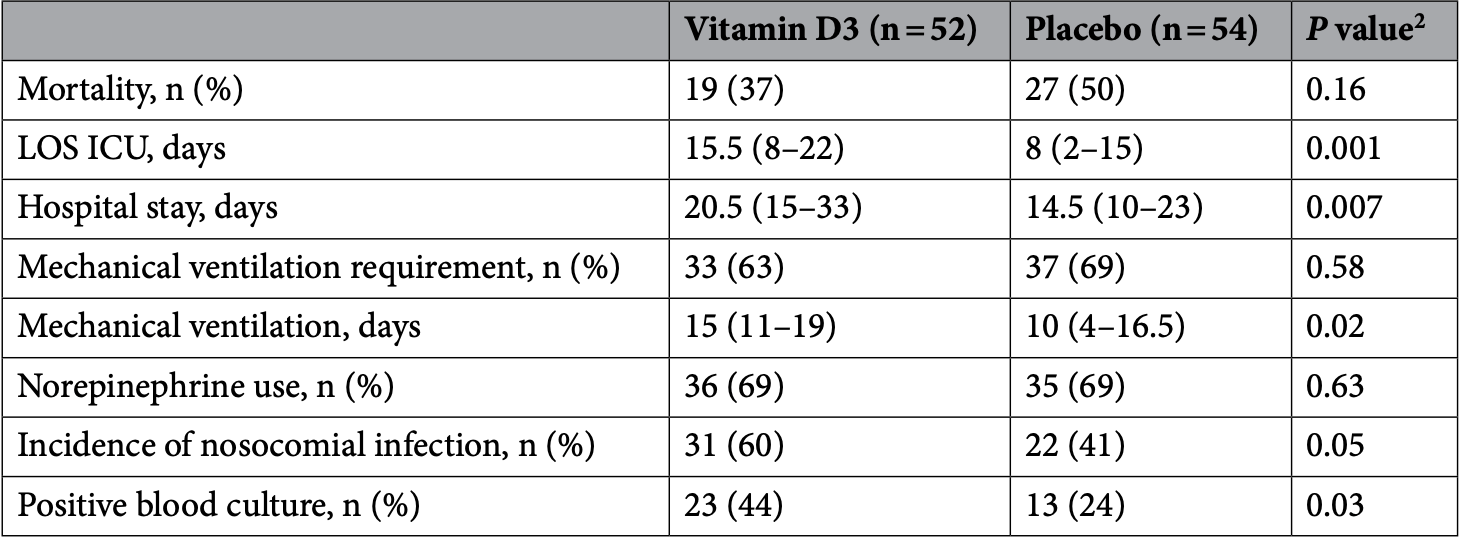
RCT ICU patients in Russia, showing significantly increased lymphocyte counts with treatment. Mortality was lower but without statistical significance. 40% of patients were on mechanical ventilation at baseline in the treatment group, compared to 30% in the placebo group.
Authors state that there has been 6 RCTs for COVID-19 and vitamin D, however there was at least 23 at the time of publication:1.
Cholecalciferol was used in this study.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 44% [33‑53%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
This is the 29th of 40 COVID-19 RCTs for vitamin D, which collectively show efficacy with p=0.0000001.
This is the 105th of 136 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
This study is excluded in the after exclusion results of meta-analysis:
very late stage study using cholecalciferol instead of calcifediol or calcitriol.
|
risk of death, 26.9% lower, RR 0.73, p = 0.18, treatment 19 of 52 (36.5%), control 27 of 54 (50.0%), NNT 7.4.
|
|
risk of mechanical ventilation, 7.4% lower, RR 0.93, p = 0.68, treatment 33 of 52 (63.5%), control 37 of 54 (68.5%), NNT 20.
|
|
ICU time, 93.8% higher, relative time 1.94, p = 0.001, treatment 52, control 54.
|
|
hospitalization time, 41.4% higher, relative time 1.41, p = 0.007, treatment 52, control 54.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bychinin et al., 3 Nov 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Russia, peer-reviewed, 7 authors, study period 1 May, 2020 - 31 January, 2022, average treatment delay 9.0 days, dosage 60,000IU day 1, 5,000IU days 2-7, 8, 5,000IU days 9-14, 15, 5,000IU days 16-21, 22, 5,000IU days 23-28, trial NCT05092698 (history) (COVID-VIT).
Contact: drbychinin@gmail.com.
Effect of vitamin D3 supplementation on cellular immunity and inflammatory markers in COVID-19 patients admitted to the ICU
Scientific Reports, doi:10.1038/s41598-022-22045-y
Vitamin D as an immunomodulator has not been studied in patients with severe COVID-19. This study aimed to estimate the efficacy of vitamin D3 supplementation on cellular immunity and inflammatory markers in patients with COVID-19 admitted to the intensive care unit (ICU). A single-center, doubleblind, randomized, placebo-controlled pilot trial was conducted (N = 110). Patients were randomly assigned to receive a weekly oral dose of 60,000 IU of vitamin D3 followed by daily maintenance doses of 5000 IU (n = 55) or placebo (n = 55). Primary outcomes were lymphocyte counts, natural killer (NK) and natural killer T (NKT) cell counts, neutrophil-to-lymphocyte ratio (NLR), and serum levels of inflammatory markers on 7th day of treatment. On day 7, patients in the vitamin D3 group displayed significantly higher NK and NKT cell counts and NLR than those in the placebo group did. The mortality rate (37% vs 50%, P = 0.16), need for mechanical ventilation (63% vs 69%, P = 0.58), incidence of nosocomial infection (60% vs 41%, P = 0.05) did not significantly differ between groups. Vitamin D3 supplementation, compared with placebo, significantly increased lymphocyte counts, but did not translate into reduced mortality in ICU. Trial Registration: ClinicalTrials.gov Identifier: NCT05092698.
Author contributions M.V.B., T.V.K., V.P.B. and A.V.T. designed research; M.V.B. conducted research and wrote the main manuscript text; T.V.K. and I.A.M. edited the paper; N.A.K. and G.M.Y. performed laboratory analyses. I.A.M. analyzed data and prepared figures; M.V.B. had primary responsibility for final content. All authors reviewed the manuscript.
Competing interests The authors declare no competing interests.
References
Al-Jaderi, Maghazachi, Effects of vitamin D3, calcipotriol and FTY720 on the expression of surface molecules and cytolytic activities of human natural killer cells and dendritic cells, Toxins
Almerighi, 1α,25-dihydroxyvitamin D3 inhibits CD40L-induced proinflammatory and immunomodulatory activity in human monocytes, Cytokine
Amrein, Papinutti, Mathew, Vila, Parekh, Vitamin D and critical illness: What endocrinology can learn from intensive care and vice versa, Endocr. Connect
Bychinin, Low circulating vitamin D in intensive care unit-admitted COVID-19 patients as a predictor of negative outcomes, J. Nutr
Castillo, Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J. Steroid Biochem. Mol. Biol
Charoenngam, Holick, Immunologic effects of vitamin D on human health and disease, Nutrients
Gönen, Rapid and effective vitamin D supplementation may present better clinical outcomes in COVID-19 (SARS-CoV-2) patients by altering serum INOS1, IL1B, IFNg, cathelicidin-LL37, and ICAM1, Nutrients
Heart, Lung, Blood, Early high-dose vitamin D 3 for critically ill, vitamin D-deficient patients, N. Engl. J. Med
Herr, Shaykhiev, Bals, The role of cathelicidin and defensins in pulmonary inflammatory diseases, Expert Opin. Biol. Ther
Hiemstra, Casian, Boraks, Jayne, Schoenmakers, Plasma exchange induces vitamin D deficiency, QJM
Holick, Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline, J. Clin. Endocrinol. Metab
Jiang, T-cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of coronavirus disease 2019, J. Infect. Dis
Jolliffe, Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials, Lancet Diabetes Endocrinol
Kalicińska, Immunosuppression as a hallmark of critical COVID-19: Prospective study, Cells
Kim, Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease, Nat. Med
Kitajima, Maruyama, Matsubara, Osame, Igata, Immune dysfunction in hypophosphatemic vitamin D-resistant rickets: Immunoregulatory reaction of 1 alpha(OH) vitamin D3, Clin. Immunol. Immunopathol
Krishnan, Acute fluid shifts influence the assessment of serum vitamin D status in critically ill patients, Crit. Care
Lakkireddy, Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID 19 disease, Sci. Rep
Lee, Differential effect of dietary vitamin D supplementation on natural killer cell activity in lean and obese mice, J. Nutr. Biochem
Lee, VDUP1 is required for the development of natural killer cells, Immunity
Lips, Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society, Eur. J. Endocrinol
Liu, Decreased T cell populations contribute to the increased severity of COVID-19, Clin. Chim. Acta
Loucera, Real world evidence of calcifediol or vitamin D prescription and mortality rate of COVID-19 in a retrospective cohort of hospitalized Andalusian patients, Sci. Rep
Martens, Gysemans, Verstuyf, Mathieu, Vitamin D's effect on immune function, Nutrients
Mazzoni, Impaired immune cell cytotoxicity in severe COVID-19 Is IL-6 dependent, J. Clin. Investig
Meckiff, Imbalance of regulatory and cytotoxic SARS-CoV-2-reactive CD4+ T cells in COVID-19, Cell
Murai, Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: A randomized clinical trial, JAMA
Ni, Immunological perspectives on the pathogenesis, diagnosis, prevention and treatment of COVID-19, Mol. Biomed
Quesada, The effect of calcitriol on natural killer cell activity in hemodialyzed patients, J. Steroid Biochem
Rastogi, Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study), Postgrad. Med. J
Sabico, Effects of a 2-week 5000 iu versus 1000 iu vitamin d3 supplementation on recovery of symptoms in patients with mild to moderate covid-19: A randomized clinical trial, Nutrients
Sun, Cytokine storm intervention in the early stages of COVID-19 pneumonia, Cytokine Growth Factor Rev
Sánchez-Zuno, Vitamin D levels in COVID-19 outpatients from western Mexico: Clinical correlation and effect of its supplementation, J. Clin. Med
Tuohimaa, Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: A longitudinal, nested case-control study in the Nordic countries, Int. J. Cancer
Vabret, Immunology of COVID-19: Current state of the science, Immunity
Varikasuvu, COVID-19 and vitamin D (Co-VIVID study): A systematic review and meta-analysis of randomized controlled trials, Expert Rev. Anti Infect Ther
Vassiliou, Vitamin D deficiency correlates with a reduced number of natural killer cells in intensive care unit (ICU) and non-ICU patients with COVID-19 pneumonia, Hellenic J. Cardiol
Weeres, The effects of 1,25-dihydroxyvitamin D3 on in vitro human NK cell development from hematopoietic stem cells, J. Immunol
Yu, Cantorna, The vitamin D receptor is required for iNKT cell development, Proc. Natl. Acad. Sci. U.S.A
Zhang, Wan, Sun, Kan, Wang, Association between vitamin D deficiency and mortality in critically ill adult patients: A meta-analysis of cohort studies, Crit. Care
Zingaropoli, Major reduction of NKT cells in patients with severe COVID-19 pneumonia, Clin. Immunol
DOI record:
{
"DOI": "10.1038/s41598-022-22045-y",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-022-22045-y",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Vitamin D as an immunomodulator has not been studied in patients with severe COVID-19. This study aimed to estimate the efficacy of vitamin D3 supplementation on cellular immunity and inflammatory markers in patients with COVID-19 admitted to the intensive care unit (ICU). A single-center, double-blind, randomized, placebo-controlled pilot trial was conducted (N = 110). Patients were randomly assigned to receive a weekly oral dose of 60,000 IU of vitamin D3 followed by daily maintenance doses of 5000 IU (n = 55) or placebo (n = 55). Primary outcomes were lymphocyte counts, natural killer (NK) and natural killer T (NKT) cell counts, neutrophil-to-lymphocyte ratio (NLR), and serum levels of inflammatory markers on 7th day of treatment. On day 7, patients in the vitamin D3 group displayed significantly higher NK and NKT cell counts and NLR than those in the placebo group did. The mortality rate (37% vs 50%, P = 0.16), need for mechanical ventilation (63% vs 69%, P = 0.58), incidence of nosocomial infection (60% vs 41%, P = 0.05) did not significantly differ between groups. Vitamin D3 supplementation, compared with placebo, significantly increased lymphocyte counts, but did not translate into reduced mortality in ICU.</jats:p><jats:p>Trial Registration: ClinicalTrials.gov Identifier: NCT05092698.</jats:p>",
"alternative-id": [
"22045"
],
"article-number": "18604",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "11 May 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "7 October 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "3 November 2022"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Bychinin",
"given": "Mikhail V.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Klypa",
"given": "Tatiana V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mandel",
"given": "Irina A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yusubalieva",
"given": "Gaukhar M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baklaushev",
"given": "Vladimir P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kolyshkina",
"given": "Nadezhda A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Troitsky",
"given": "Aleksandr V.",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct05092698",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
11,
3
]
],
"date-time": "2022-11-03T15:04:41Z",
"timestamp": 1667487881000
},
"deposited": {
"date-parts": [
[
2022,
11,
3
]
],
"date-time": "2022-11-03T15:16:58Z",
"timestamp": 1667488618000
},
"indexed": {
"date-parts": [
[
2022,
11,
4
]
],
"date-time": "2022-11-04T04:57:51Z",
"timestamp": 1667537871568
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
11,
3
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
3
]
],
"date-time": "2022-11-03T00:00:00Z",
"timestamp": 1667433600000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
3
]
],
"date-time": "2022-11-03T00:00:00Z",
"timestamp": 1667433600000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-022-22045-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-22045-y",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-22045-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2022,
11,
3
]
]
},
"published-online": {
"date-parts": [
[
2022,
11,
3
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1186/s43556-020-00015-y",
"author": "Y Ni",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Mol. Biomed.",
"key": "22045_CR1",
"unstructured": "Ni, Y. et al. Immunological perspectives on the pathogenesis, diagnosis, prevention and treatment of COVID-19. Mol. Biomed. 2, 1 (2021).",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1172/JCI138554",
"author": "A Mazzoni",
"doi-asserted-by": "crossref",
"first-page": "4694",
"journal-title": "J. Clin. Investig.",
"key": "22045_CR2",
"unstructured": "Mazzoni, A. et al. Impaired immune cell cytotoxicity in severe COVID-19 Is IL-6 dependent. J. Clin. Investig. 130, 4694–4703 (2020).",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.10.001",
"author": "BJ Meckiff",
"doi-asserted-by": "crossref",
"first-page": "1340",
"journal-title": "Cell",
"key": "22045_CR3",
"unstructured": "Meckiff, B. J. et al. Imbalance of regulatory and cytotoxic SARS-CoV-2-reactive CD4+ T cells in COVID-19. Cell 183, 1340–1353 (2020).",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1016/j.cca.2020.05.019",
"author": "R Liu",
"doi-asserted-by": "crossref",
"first-page": "110",
"journal-title": "Clin. Chim. Acta",
"key": "22045_CR4",
"unstructured": "Liu, R. et al. Decreased T cell populations contribute to the increased severity of COVID-19. Clin. Chim. Acta 508, 110–114 (2020).",
"volume": "508",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiaa252",
"author": "M Jiang",
"doi-asserted-by": "crossref",
"first-page": "198",
"journal-title": "J. Infect. Dis.",
"key": "22045_CR5",
"unstructured": "Jiang, M. et al. T-cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of coronavirus disease 2019. J. Infect. Dis. 222, 198–202 (2020).",
"volume": "222",
"year": "2020"
},
{
"DOI": "10.3390/nu12072097",
"author": "N Charoenngam",
"doi-asserted-by": "crossref",
"first-page": "2097",
"journal-title": "Nutrients",
"key": "22045_CR6",
"unstructured": "Charoenngam, N. & Holick, M. F. Immunologic effects of vitamin D on human health and disease. Nutrients 12, 2097 (2020).",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1517/14712598.7.9.1449",
"author": "C Herr",
"doi-asserted-by": "crossref",
"first-page": "1449",
"journal-title": "Expert Opin. Biol. Ther.",
"key": "22045_CR7",
"unstructured": "Herr, C., Shaykhiev, R. & Bals, R. The role of cathelicidin and defensins in pulmonary inflammatory diseases. Expert Opin. Biol. Ther. 7, 1449–1461 (2007).",
"volume": "7",
"year": "2007"
},
{
"DOI": "10.3390/nu12051248",
"author": "PJ Martens",
"doi-asserted-by": "crossref",
"first-page": "1248",
"journal-title": "Nutrients",
"key": "22045_CR8",
"unstructured": "Martens, P. J., Gysemans, C., Verstuyf, A. & Mathieu, A. C. Vitamin D’s effect on immune function. Nutrients 12, 1248 (2020).",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1186/s13054-014-0684-9",
"author": "YP Zhang",
"doi-asserted-by": "crossref",
"first-page": "684",
"journal-title": "Crit. Care",
"key": "22045_CR9",
"unstructured": "Zhang, Y. P., Wan, Y. D., Sun, T. W., Kan, Q. C. & Wang, L. X. Association between vitamin D deficiency and mortality in critically ill adult patients: A meta-analysis of cohort studies. Crit. Care 18, 684 (2014).",
"volume": "18",
"year": "2014"
},
{
"DOI": "10.1093/jn/nxab107",
"author": "MV Bychinin",
"doi-asserted-by": "crossref",
"first-page": "2199",
"journal-title": "J. Nutr.",
"key": "22045_CR10",
"unstructured": "Bychinin, M. V. et al. Low circulating vitamin D in intensive care unit-admitted COVID-19 patients as a predictor of negative outcomes. J. Nutr. 151, 2199–2205 (2021).",
"volume": "151",
"year": "2021"
},
{
"DOI": "10.3390/nu13114047",
"author": "MS Gönen",
"doi-asserted-by": "crossref",
"first-page": "4047",
"journal-title": "Nutrients",
"key": "22045_CR11",
"unstructured": "Gönen, M. S. et al. Rapid and effective vitamin D supplementation may present better clinical outcomes in COVID-19 (SARS-CoV-2) patients by altering serum INOS1, IL1B, IFNg, cathelicidin-LL37, and ICAM1. Nutrients 13, 4047 (2021).",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/S2213-8587(21)00051-6",
"author": "DA Jolliffe",
"doi-asserted-by": "crossref",
"first-page": "276",
"journal-title": "Lancet Diabetes Endocrinol.",
"key": "22045_CR12",
"unstructured": "Jolliffe, D. A. et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 9, 276–292 (2021).",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.cyto.2008.12.009",
"author": "C Almerighi",
"doi-asserted-by": "crossref",
"first-page": "190",
"issue": "3",
"journal-title": "Cytokine",
"key": "22045_CR13",
"unstructured": "Almerighi, C. et al. 1α,25-dihydroxyvitamin D3 inhibits CD40L-induced proinflammatory and immunomodulatory activity in human monocytes. Cytokine 45(3), 190–197 (2009).",
"volume": "45",
"year": "2009"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"author": "M Entrenas Castillo",
"doi-asserted-by": "crossref",
"journal-title": "J. Steroid Biochem. Mol. Biol.",
"key": "22045_CR14",
"unstructured": "Entrenas Castillo, M. et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 203, 105751 (2020).",
"volume": "203",
"year": "2020"
},
{
"DOI": "10.1038/s41598-020-79139-8",
"author": "M Lakkireddy",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Sci. Rep.",
"key": "22045_CR15",
"unstructured": "Lakkireddy, M. et al. Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID 19 disease. Sci. Rep. 11, 1–8 (2021).",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.26848",
"author": "IH Murai",
"doi-asserted-by": "crossref",
"first-page": "1053",
"journal-title": "JAMA",
"key": "22045_CR16",
"unstructured": "Murai, I. H. et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: A randomized clinical trial. JAMA 325, 1053–1060 (2021).",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.3390/nu13072170",
"author": "S Sabico",
"doi-asserted-by": "crossref",
"first-page": "2170",
"journal-title": "Nutrients",
"key": "22045_CR17",
"unstructured": "Sabico, S. et al. Effects of a 2-week 5000 iu versus 1000 iu vitamin d3 supplementation on recovery of symptoms in patients with mild to moderate covid-19: A randomized clinical trial. Nutrients 13, 2170 (2021).",
"volume": "13",
"year": "2021"
},
{
"author": "A Rastogi",
"first-page": "87",
"journal-title": "Postgrad. Med. J.",
"key": "22045_CR18",
"unstructured": "Rastogi, A. et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study). Postgrad. Med. J. 98, 87–90 (2022).",
"volume": "98",
"year": "2022"
},
{
"DOI": "10.3390/jcm10112378",
"author": "GA Sánchez-Zuno",
"doi-asserted-by": "crossref",
"first-page": "2378",
"journal-title": "J. Clin. Med.",
"key": "22045_CR19",
"unstructured": "Sánchez-Zuno, G. A. et al. Vitamin D levels in COVID-19 outpatients from western Mexico: Clinical correlation and effect of its supplementation. J. Clin. Med. 10, 2378 (2021).",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1080/14787210.2022.2035217",
"author": "SR Varikasuvu",
"doi-asserted-by": "crossref",
"first-page": "907",
"journal-title": "Expert Rev. Anti Infect Ther.",
"key": "22045_CR20",
"unstructured": "Varikasuvu, S. R. et al. COVID-19 and vitamin D (Co-VIVID study): A systematic review and meta-analysis of randomized controlled trials. Expert Rev. Anti Infect Ther. 20, 907–913 (2022).",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1016/0090-1229(89)90097-4",
"author": "I Kitajima",
"doi-asserted-by": "crossref",
"first-page": "24",
"journal-title": "Clin. Immunol. Immunopathol.",
"key": "22045_CR21",
"unstructured": "Kitajima, I., Maruyama, I., Matsubara, H., Osame, M. & Igata, A. Immune dysfunction in hypophosphatemic vitamin D-resistant rickets: Immunoregulatory reaction of 1 alpha(OH) vitamin D3. Clin. Immunol. Immunopathol. 53, 24–31 (1989).",
"volume": "53",
"year": "1989"
},
{
"DOI": "10.1016/0022-4731(89)90120-9",
"author": "JM Quesada",
"doi-asserted-by": "crossref",
"first-page": "423",
"journal-title": "J. Steroid Biochem.",
"key": "22045_CR22",
"unstructured": "Quesada, J. M. et al. The effect of calcitriol on natural killer cell activity in hemodialyzed patients. J. Steroid Biochem. 34, 423–425 (1989).",
"volume": "34",
"year": "1989"
},
{
"DOI": "10.1073/pnas.0711558105",
"author": "S Yu",
"doi-asserted-by": "crossref",
"first-page": "5207",
"journal-title": "Proc. Natl. Acad. Sci. U.S.A.",
"key": "22045_CR23",
"unstructured": "Yu, S. & Cantorna, M. T. The vitamin D receptor is required for iNKT cell development. Proc. Natl. Acad. Sci. U.S.A. 105, 5207–5212 (2008).",
"volume": "105",
"year": "2008"
},
{
"DOI": "10.1016/j.jnutbio.2018.01.004",
"author": "GY Lee",
"doi-asserted-by": "crossref",
"first-page": "178",
"journal-title": "J. Nutr. Biochem.",
"key": "22045_CR24",
"unstructured": "Lee, G. Y. et al. Differential effect of dietary vitamin D supplementation on natural killer cell activity in lean and obese mice. J. Nutr. Biochem. 55, 178–184 (2018).",
"volume": "55",
"year": "2018"
},
{
"DOI": "10.4049/jimmunol.1400698",
"author": "MA Weeres",
"doi-asserted-by": "crossref",
"first-page": "3456",
"journal-title": "J. Immunol.",
"key": "22045_CR25",
"unstructured": "Weeres, M. A. et al. The effects of 1,25-dihydroxyvitamin D3 on in vitro human NK cell development from hematopoietic stem cells. J. Immunol. 193, 3456–3462 (2014).",
"volume": "193",
"year": "2014"
},
{
"DOI": "10.1016/j.immuni.2004.12.012",
"author": "KN Lee",
"doi-asserted-by": "crossref",
"first-page": "195",
"journal-title": "Immunity",
"key": "22045_CR26",
"unstructured": "Lee, K. N. et al. VDUP1 is required for the development of natural killer cells. Immunity 22, 195–208 (2005).",
"volume": "22",
"year": "2005"
},
{
"DOI": "10.3390/toxins5111932",
"author": "Z Al-Jaderi",
"doi-asserted-by": "crossref",
"first-page": "1932",
"journal-title": "Toxins (Basel)",
"key": "22045_CR27",
"unstructured": "Al-Jaderi, Z. & Maghazachi, A. A. Effects of vitamin D3, calcipotriol and FTY720 on the expression of surface molecules and cytolytic activities of human natural killer cells and dendritic cells. Toxins (Basel) 5, 1932–1947 (2013).",
"volume": "5",
"year": "2013"
},
{
"DOI": "10.3390/cells10061293",
"author": "E Kalicińska",
"doi-asserted-by": "crossref",
"first-page": "1293",
"journal-title": "Cells",
"key": "22045_CR28",
"unstructured": "Kalicińska, E. et al. Immunosuppression as a hallmark of critical COVID-19: Prospective study. Cells 10, 1293 (2021).",
"volume": "10",
"year": "2021"
},
{
"author": "MA Zingaropoli",
"journal-title": "Clin. Immunol.",
"key": "22045_CR29",
"unstructured": "Zingaropoli, M. A. et al. Major reduction of NKT cells in patients with severe COVID-19 pneumonia. Clin. Immunol. 22, 108630 (2021).",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1038/nm1770",
"author": "EY Kim",
"doi-asserted-by": "crossref",
"first-page": "633",
"journal-title": "Nat. Med.",
"key": "22045_CR30",
"unstructured": "Kim, E. Y. et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat. Med. 14, 633–640 (2008).",
"volume": "14",
"year": "2008"
},
{
"DOI": "10.1016/j.immuni.2020.05.002",
"author": "N Vabret",
"doi-asserted-by": "crossref",
"first-page": "910",
"journal-title": "Immunity",
"key": "22045_CR31",
"unstructured": "Vabret, N. et al. Immunology of COVID-19: Current state of the science. Immunity 52, 910–941 (2020).",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.1016/j.hjc.2020.11.011",
"author": "AG Vassiliou",
"doi-asserted-by": "crossref",
"first-page": "381",
"journal-title": "Hellenic J. Cardiol.",
"key": "22045_CR32",
"unstructured": "Vassiliou, A. G. et al. Vitamin D deficiency correlates with a reduced number of natural killer cells in intensive care unit (ICU) and non-ICU patients with COVID-19 pneumonia. Hellenic J. Cardiol. 62, 381–383 (2021).",
"volume": "62",
"year": "2021"
},
{
"DOI": "10.1530/EC-18-0184",
"author": "K Amrein",
"doi-asserted-by": "crossref",
"first-page": "304",
"journal-title": "Endocr. Connect.",
"key": "22045_CR33",
"unstructured": "Amrein, K., Papinutti, A., Mathew, E., Vila, G. & Parekh, D. Vitamin D and critical illness: What endocrinology can learn from intensive care and vice versa. Endocr. Connect. 7, 304–315 (2018).",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1186/cc9341",
"author": "A Krishnan",
"doi-asserted-by": "crossref",
"first-page": "216",
"journal-title": "Crit. Care",
"key": "22045_CR34",
"unstructured": "Krishnan, A. et al. Acute fluid shifts influence the assessment of serum vitamin D status in critically ill patients. Crit. Care 14, 216 (2010).",
"volume": "14",
"year": "2010"
},
{
"DOI": "10.1093/qjmed/hct208",
"author": "TF Hiemstra",
"doi-asserted-by": "crossref",
"first-page": "123",
"journal-title": "QJM",
"key": "22045_CR35",
"unstructured": "Hiemstra, T. F., Casian, A., Boraks, P., Jayne, D. R. & Schoenmakers, I. Plasma exchange induces vitamin D deficiency. QJM 107, 123–130 (2014).",
"volume": "107",
"year": "2014"
},
{
"DOI": "10.1038/s41598-021-02701-5",
"author": "C Loucera",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Rep.",
"key": "22045_CR36",
"unstructured": "Loucera, C. et al. Real world evidence of calcifediol or vitamin D prescription and mortality rate of COVID-19 in a retrospective cohort of hospitalized Andalusian patients. Sci. Rep. 11, 23380 (2021).",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa1911124",
"author": "AA Ginde",
"doi-asserted-by": "crossref",
"first-page": "2529",
"journal-title": "N. Engl. J. Med.",
"key": "22045_CR37",
"unstructured": "National Heart, Lung, and Blood Institute PETAL Clinical Trials Network et al. Early high-dose vitamin D3 for critically ill, vitamin D-deficient patients. N. Engl. J. Med. 381, 2529–2540 (2019).",
"volume": "381",
"year": "2019"
},
{
"DOI": "10.1002/ijc.11375",
"author": "P Tuohimaa",
"doi-asserted-by": "crossref",
"first-page": "104",
"journal-title": "Int. J. Cancer",
"key": "22045_CR38",
"unstructured": "Tuohimaa, P. et al. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: A longitudinal, nested case-control study in the Nordic countries. Int. J. Cancer 108, 104–108 (2004).",
"volume": "108",
"year": "2004"
},
{
"DOI": "10.1016/j.cytogfr.2020.04.002",
"author": "X Sun",
"doi-asserted-by": "crossref",
"first-page": "38",
"journal-title": "Cytokine Growth Factor Rev.",
"key": "22045_CR39",
"unstructured": "Sun, X. et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 53, 38–42 (2020).",
"volume": "53",
"year": "2020"
},
{
"DOI": "10.1210/jc.2011-0385",
"author": "MF Holick",
"doi-asserted-by": "crossref",
"first-page": "1911",
"journal-title": "J. Clin. Endocrinol. Metab.",
"key": "22045_CR40",
"unstructured": "Holick, M. F. et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 96, 1911–1930 (2011).",
"volume": "96",
"year": "2011"
},
{
"DOI": "10.1530/EJE-18-0736",
"author": "P Lips",
"doi-asserted-by": "crossref",
"first-page": "23",
"journal-title": "Eur. J. Endocrinol.",
"key": "22045_CR41",
"unstructured": "Lips, P. et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 180, 23–54 (2019).",
"volume": "180",
"year": "2019"
}
],
"reference-count": 41,
"references-count": 41,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-022-22045-y"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Effect of vitamin D3 supplementation on cellular immunity and inflammatory markers in COVID-19 patients admitted to the ICU",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "12"
}
