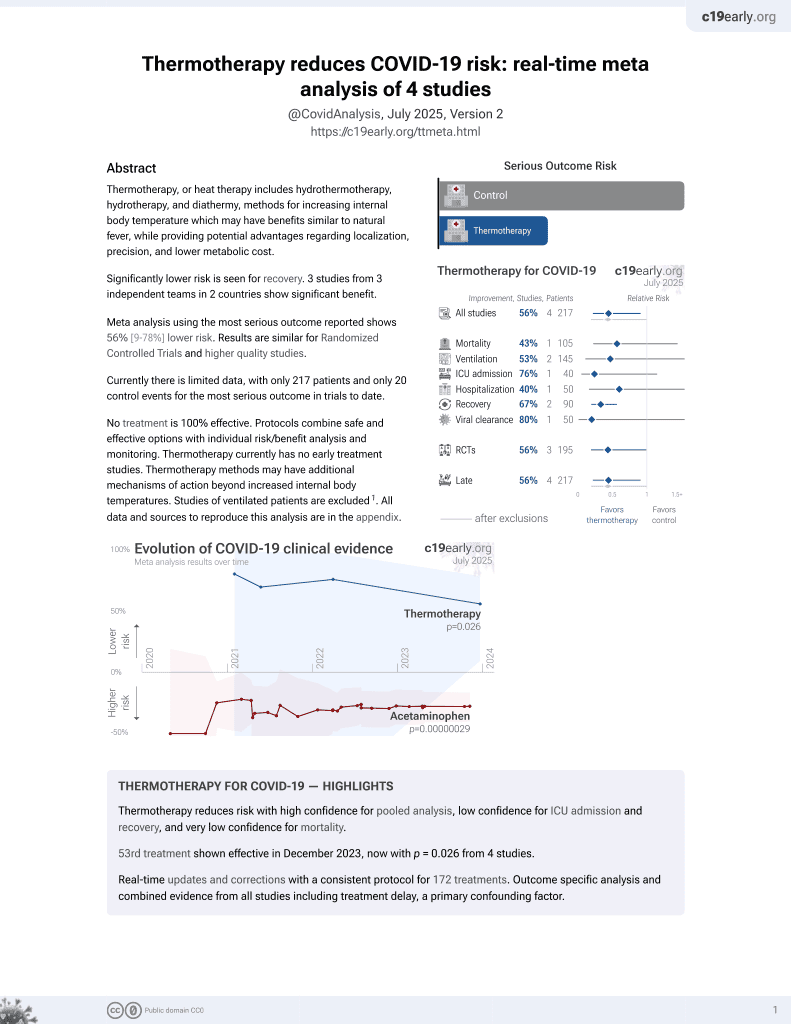
Core Warming of Coronavirus Disease 2019 Patients Undergoing Mechanical Ventilation: A Pilot Study
et al., Therapeutic Hypothermia and Temperature Management, doi:10.1089/ther.2023.0030, NCT04494867, Nov 2023
54th treatment shown to reduce risk in
December 2023, now with p = 0.026 from 4 studies.
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
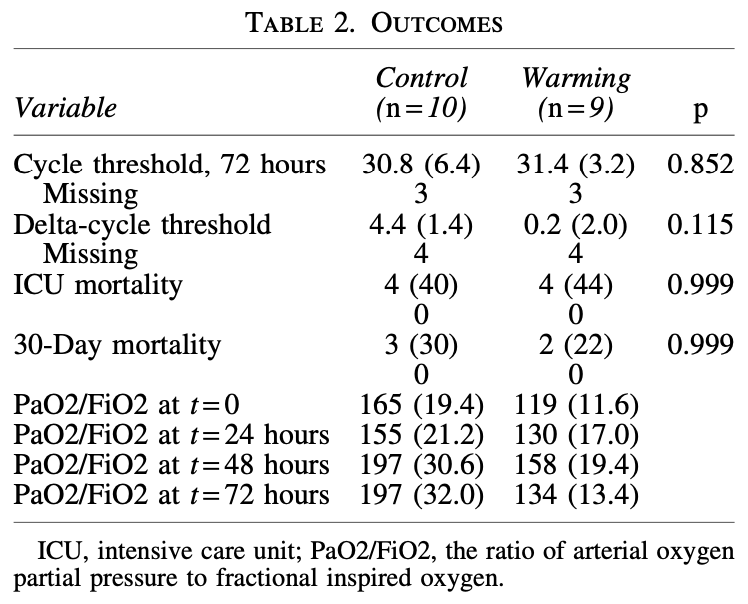
RCT of 19 mechanically ventilated COVID-19 patients randomized to standard care or core warming with an esophageal heat exchanger to target 39.8C for 72 hours. The core warming group reached higher temperatures but had similar outcomes including PaO2/FiO2 ratios, viral clearance, duration of ventilation, and mortality. Baseline SOFA and PaO2/FiO2 indicate higher severity in the treatment group. The results suggest that core warming is feasible and safe but a larger trial with a multimodal warming strategy may be needed to optimize time to target temperature and further evaluate effects.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
This study is excluded in meta-analysis:
very late treatment, mechanically ventilated patients, baseline SOFA and PaO2/FiO2 show higher severity in the treatment group; very late stage, ventilated patients.
|
risk of death, 11.1% higher, RR 1.11, p = 1.00, treatment 4 of 9 (44.4%), control 4 of 10 (40.0%).
|
|
risk of death, 25.9% lower, RR 0.74, p = 1.00, treatment 2 of 9 (22.2%), control 3 of 10 (30.0%), NNT 13, day 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bonfanti et al., 30 Nov 2023, Randomized Controlled Trial, USA, peer-reviewed, mean age 60.5, 8 authors, study period September 2020 - February 2022, average treatment delay 9.4 days, trial NCT04494867 (history).
Core Warming of Coronavirus Disease 2019 Patients Undergoing Mechanical Ventilation: A Pilot Study
Therapeutic Hypothermia and Temperature Management, doi:10.1089/ther.2023.0030
Fever is a recognized protective factor in patients with sepsis, and growing data suggest beneficial effects on outcomes in sepsis with elevated temperature, with a recent pilot randomized controlled trial (RCT) showing lower mortality by warming afebrile sepsis patients in the intensive care unit (ICU). The objective of this prospective single-site RCT was to determine if core warming improves respiratory physiology of mechanically ventilated patients with coronavirus disease 2019 (COVID-19), allowing earlier weaning from ventilation, and greater overall survival. A total of 19 patients with mean age of 60.5 (-12.5) years, 37% female, mean weight 95.1 (-18.6) kg, and mean body mass index 34.5 (-5.9) kg/m 2 with COVID-19 requiring mechanical ventilation were enrolled from September 2020 to February 2022. Patients were randomized 1:1 to standard of care or to receive core warming for 72 hours through an esophageal heat exchanger commonly utilized in critical care and surgical patients. The maximum target temperature was 39.8°C. A total of 10 patients received usual care and 9 patients received esophageal core warming. After 72 hours of warming, the ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) ratios were 197 (-32) and 134 (-13.4), cycle thresholds were 30.8 (-6.4) and 31.4 (-3.2), ICU mortalities were 40% and 44%, 30-day mortalities were 30% and 22%, and mean 30-day ventilator-free days were 11.9 (-12.6) and 6.8 (-10.2) for standard of care and warmed patients, respectively ( p = NS). This pilot study suggests that core warming of patients with COVID-19 undergoing mechanical ventilation is feasible and appears safe. Optimizing time to achieve febrile-range temperature may require a multimodal temperature management strategy to further evaluate effects on outcome. ClinicalTrials.
Authors' Contributions Writing-original draft, review, and editing by N.P.B. Methodology, data curation, and formal analysis by N.M.M.
References
Anderson, Joseph, Fisher, Targeted temperature management using esophageal cooling, Ther Hypothermia Temp Manag, doi:10.1089/ther.2022.0033
Arabi, Myatra, Lobo, Surging ICU during COVID-19 pandemic: An overview, Curr Opin Crit Care, doi:10.1097/mcc.0000000000001001
Bonfanti, Gundert, Drewry, Core warming of coronavirus disease 2019 (COVID-19) patients undergoing mechanical ventilation-A protocol for a randomized controlled pilot study, PLoS One, doi:10.1371/journal.pone.0243190
Bonfanti, None
Brandts, Ndjave, Graninger, Effect of paracetamol on parasite clearance time in Plasmodium falciparum malaria, Lancet, doi:10.1016/s0140-6736(97)02255-1
Chan, Peiris, Lam, The effects of temperature and relative humidity on the viability of the SARS coronavirus, Adv Virol, doi:10.1155/2011/734690
Dallimore, Ebmeier, Thayabaran, Effect of active temperature management on mortality in intensive care unit patients, Crit Care Resusc
Doran, Angelis, Baumgardner, Acetaminophen: More harm than good for chickenpox?, J Pediatr, doi:10.1016/s0022-3476(89)80461-5
Drewry, Ablordeppey, Murray, Antipyretic therapy in critically ill septic patients: A systematic review and meta-analysis, Crit Care Med, doi:10.1097/CCM.0000000000002285
Drewry, Ablordeppey, Murray, Monocyte function and clinical outcomes in febrile and afebrile patients with severe sepsis, Shock, doi:10.1097/SHK.0000000000001083
Drewry, Hotchkiss, Counterpoint: Should antipyretic therapy be given routinely to febrile patients in septic shock? No, Chest, doi:10.1378/chest.13-0918
Drewry, Mohr, Ablordeppey, Therapeutic hyperthermia is associated with improved survival in afebrile critically ill patients with sepsis: A pilot randomized trial, Crit Care Med, doi:10.1097/ccm.0000000000005470
Evans, Repasky, Fisher, Fever and the thermal regulation of immunity: The immune system feels the heat, Nat Rev Immunol, doi:10.1038/nri3843
Foxman, Storer, Fitzgerald, Temperaturedependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells, Proc Natl Acad Sci, doi:10.1073/pnas.1411030112
Furrer, Garcia, Pfister, Perioperative targeted temperature management of severely burned patients by means of an oesophageal temperature probe, Burns, doi:10.1016/j.burns.2022.03.015
Gozzoli, Schottker, Suter, Is it worth treating fever in intensive care unit patients? Preliminary results from 228 BONFANTI ET AL. a randomized trial of the effect of external cooling, Arch Intern Med, doi:10.1001/archinte.161.1.121
Itenov, Johansen, Bestle, Induced hypothermia in patients with septic shock and respiratory failure (CASS): A randomised, controlled, open-label trial, Lancet Respir Med, doi:10.1016/s2213-2600(18)30004-3
Kwei-Nsoro, Attar, Shaka, Independent predictors and causes of thirty-day gastrointestinal readmissions following COVID-19-related hospitalizations: Analysis of the National Readmission Database, Gastroenterol Res, doi:10.14740/gr1623
Laporte, Stevaert, Raeymaekers, Hemagglutinin cleavability, acid stability, and temperature dependence optimize influenza B virus for replication in human airways, J Virol, doi:10.1128/jvi.01430-19
Launey, Nesseler, Malle ´dant, Clinical review: Fever in septic ICU patients-Friend or foe?, Crit Care, doi:10.1186/cc10097
Lee, Zhong, Mace, Elevation in body temperature to fever range enhances and prolongs subsequent responsiveness of macrophages to endotoxin challenge, PLoS One, doi:10.1371/journal.pone.0030077
Leung, Toor, Akhtar, Real-world results of oesophageal protection from a temperature control device during left atrial ablation, Europace, doi:10.1093/europace/euad099
Peters, Woolfall, Khan, Permissive versus restrictive temperature thresholds in critically ill children with fever and infection: A multicentre randomized clinical pilot trial, Crit Care, doi:10.1186/s13054-019-2354-4
Ping, Rapid response to: Graphic Outbreak of severe acute respiratory syndrome in Hong Kong Special Administrative Region: Case report, BMJ
Roger, COVID-19: Should we consider it as a septic shock? (The treatment of COVID-19 patients in the ICU), Curr Opin Anaesthesiol, doi:10.1097/aco.0000000000000956
Rumbus, Matics, Hegyi, Fever is associated with reduced, hypothermia with increased mortality in septic patients: A meta-analysis of clinical trials, PLoS One, doi:10.1371/journal.pone.0170152
Saoraya, Musikatavorn, Puttaphaisan, Intensive fever control using a therapeutic normothermia protocol in patients with febrile early septic shock: A randomized feasibility trial and exploration of the immunomodulatory effects, SAGE Open Med, doi:10.1177/2050312120928732
Schulman, Namias, Doherty, The effect of antipyretic therapy upon outcomes in critically ill patients: A randomized, prospective study, Surg Infect (Larchmt), doi:10.1089/sur.2005.6.369
Sessler, Thermoregulatory defense mechanisms, Crit Care Med, doi:10.1097/CCM.0b013e3181aa5568
Stanley, Jackson, Panusarn, Increased virus shedding with aspirin treatment of rhinovirus infection, JAMA
Wang, Xu, Gao, Detection of SARS-CoV-2 in different types of clinical specimens, JAMA, doi:10.1001/jama.2020.3786
Young, Bellomo, Bernard, Fever control in critically ill adults. An individual patient data meta-analysis of randomised controlled trials, Intensive Care Med, doi:10.1007/s00134-019-05553-w
Young, Saxena, Bellomo, Acetaminophen for fever in critically ill patients with suspected infection, N Engl J Med, doi:10.1056/NEJMoa1508375
Zhang, Antipyretic therapy in critically ill patients with established sepsis: A trial sequential analysis, PLoS One, doi:10.1371/journal.pone.0117279
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
Zou, Ruan, Huang, SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med, doi:10.1056/NEJMc2001737
DOI record:
{
"DOI": "10.1089/ther.2023.0030",
"ISSN": [
"2153-7658",
"2153-7933"
],
"URL": "http://dx.doi.org/10.1089/ther.2023.0030",
"alternative-id": [
"10.1089/ther.2023.0030"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9564-9536",
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Texas at Southwestern Medical Center, Dallas, Texas, USA."
}
],
"authenticated-orcid": false,
"family": "Bonfanti",
"given": "Nathaniel P.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA."
}
],
"family": "Mohr",
"given": "Nicholas M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care, Sharp Memorial Hospital, San Diego, California, USA."
}
],
"family": "Willms",
"given": "David C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Division of Infectious Disease, VA North Texas Health Care System and University of Texas Southwestern Medical Center, Dallas, Texas, USA."
}
],
"family": "Bedimo",
"given": "Roger J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Texas at Southwestern Medical Center, Dallas, Texas, USA."
}
],
"family": "Gundert",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Anesthesiology, University of Texas at Southwestern Medical Center, Dallas, Texas, USA."
}
],
"family": "Goff",
"given": "Kristina L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Texas at Southwestern Medical Center, Dallas, Texas, USA."
}
],
"family": "Kulstad",
"given": "Erik B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri, USA."
}
],
"family": "Drewry",
"given": "Anne M.",
"sequence": "additional"
}
],
"container-title": "Therapeutic Hypothermia and Temperature Management",
"container-title-short": "Therapeutic Hypothermia and Temperature Management",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
8,
1
]
],
"date-time": "2023-08-01T19:06:15Z",
"timestamp": 1690916775000
},
"deposited": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T18:51:21Z",
"timestamp": 1701456681000
},
"indexed": {
"date-parts": [
[
2023,
12,
25
]
],
"date-time": "2023-12-25T19:27:53Z",
"timestamp": 1703532473686
},
"is-referenced-by-count": 2,
"issue": "4",
"issued": {
"date-parts": [
[
2023,
12,
1
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2023,
12,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.liebertpub.com/nv/resources-tools/text-and-data-mining-policy/121/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
}
],
"link": [
{
"URL": "https://www.liebertpub.com/doi/full-xml/10.1089/ther.2023.0030",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.liebertpub.com/doi/pdf/10.1089/ther.2023.0030",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "278",
"original-title": [],
"page": "225-229",
"prefix": "10.1089",
"published": {
"date-parts": [
[
2023,
12,
1
]
]
},
"published-print": {
"date-parts": [
[
2023,
12,
1
]
]
},
"publisher": "Mary Ann Liebert Inc",
"reference": [
{
"DOI": "10.1089/ther.2022.0033",
"doi-asserted-by": "publisher",
"key": "B1"
},
{
"DOI": "10.1097/mcc.0000000000001001",
"doi-asserted-by": "publisher",
"key": "B2"
},
{
"DOI": "10.1371/journal.pone.0243190",
"doi-asserted-by": "publisher",
"key": "B3"
},
{
"DOI": "10.1016/s0140-6736(97)02255-1",
"doi-asserted-by": "publisher",
"key": "B4"
},
{
"DOI": "10.1155/2011/734690",
"doi-asserted-by": "publisher",
"key": "B5"
},
{
"DOI": "10.1016/S1441-2772(23)00758-5",
"doi-asserted-by": "publisher",
"key": "B6"
},
{
"DOI": "10.1016/s0022-3476(89)80461-5",
"doi-asserted-by": "publisher",
"key": "B7"
},
{
"DOI": "10.1097/CCM.0000000000002285",
"doi-asserted-by": "publisher",
"key": "B8"
},
{
"DOI": "10.1097/SHK.0000000000001083",
"doi-asserted-by": "publisher",
"key": "B9"
},
{
"DOI": "10.1378/chest.13-0918",
"doi-asserted-by": "publisher",
"key": "B10"
},
{
"DOI": "10.1097/ccm.0000000000005470",
"doi-asserted-by": "publisher",
"key": "B11"
},
{
"DOI": "10.1038/nri3843",
"doi-asserted-by": "publisher",
"key": "B12"
},
{
"DOI": "10.1073/pnas.1411030112",
"doi-asserted-by": "publisher",
"key": "B13"
},
{
"DOI": "10.1016/j.burns.2022.03.015",
"doi-asserted-by": "publisher",
"key": "B14"
},
{
"DOI": "10.1001/archinte.161.1.121",
"doi-asserted-by": "publisher",
"key": "B15"
},
{
"DOI": "10.1016/s2213-2600(18)30004-3",
"doi-asserted-by": "publisher",
"key": "B16"
},
{
"DOI": "10.14740/gr1623",
"doi-asserted-by": "publisher",
"key": "B17"
},
{
"DOI": "10.1128/JVI.01430-19",
"doi-asserted-by": "publisher",
"key": "B18"
},
{
"DOI": "10.1186/cc10097",
"doi-asserted-by": "publisher",
"key": "B19"
},
{
"DOI": "10.1371/journal.pone.0030077",
"doi-asserted-by": "publisher",
"key": "B20"
},
{
"DOI": "10.1093/europace/euad099",
"doi-asserted-by": "publisher",
"key": "B21"
},
{
"DOI": "10.1186/s13054-019-2354-4",
"doi-asserted-by": "publisher",
"key": "B22"
},
{
"DOI": "10.1136/bmj.326.7394.850",
"doi-asserted-by": "publisher",
"key": "B23"
},
{
"DOI": "10.1097/aco.0000000000000956",
"doi-asserted-by": "publisher",
"key": "B24"
},
{
"DOI": "10.1371/journal.pone.0170152",
"doi-asserted-by": "publisher",
"key": "B25"
},
{
"DOI": "10.1177/2050312120928732",
"doi-asserted-by": "publisher",
"key": "B26"
},
{
"DOI": "10.1089/sur.2005.6.369",
"doi-asserted-by": "publisher",
"key": "B27"
},
{
"DOI": "10.1097/CCM.0b013e3181aa5568",
"doi-asserted-by": "publisher",
"key": "B28"
},
{
"DOI": "10.1001/jama.1975.03240240018017",
"doi-asserted-by": "publisher",
"key": "B29"
},
{
"DOI": "10.1001/jama.2020.3786",
"doi-asserted-by": "publisher",
"key": "B30"
},
{
"DOI": "10.1056/NEJMoa1508375",
"doi-asserted-by": "publisher",
"key": "B31"
},
{
"DOI": "10.1007/s00134-019-05553-w",
"doi-asserted-by": "publisher",
"key": "B32"
},
{
"DOI": "10.1371/journal.pone.0117279",
"doi-asserted-by": "publisher",
"key": "B33"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "B34"
},
{
"DOI": "10.1056/NEJMc2001737",
"doi-asserted-by": "publisher",
"key": "B35"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.liebertpub.com/doi/10.1089/ther.2023.0030"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Anesthesiology and Pain Medicine",
"Critical Care and Intensive Care Medicine"
],
"subtitle": [],
"title": "Core Warming of Coronavirus Disease 2019 Patients Undergoing Mechanical Ventilation: A Pilot Study",
"type": "journal-article",
"volume": "13"
}