The association of prior paracetamol intake with outcome of very old intensive care patients with COVID-19: results from an international prospective multicentre trial
et al., BMC Geriatrics, doi:10.1186/s12877-022-03709-w, NCT04321265, Dec 2022
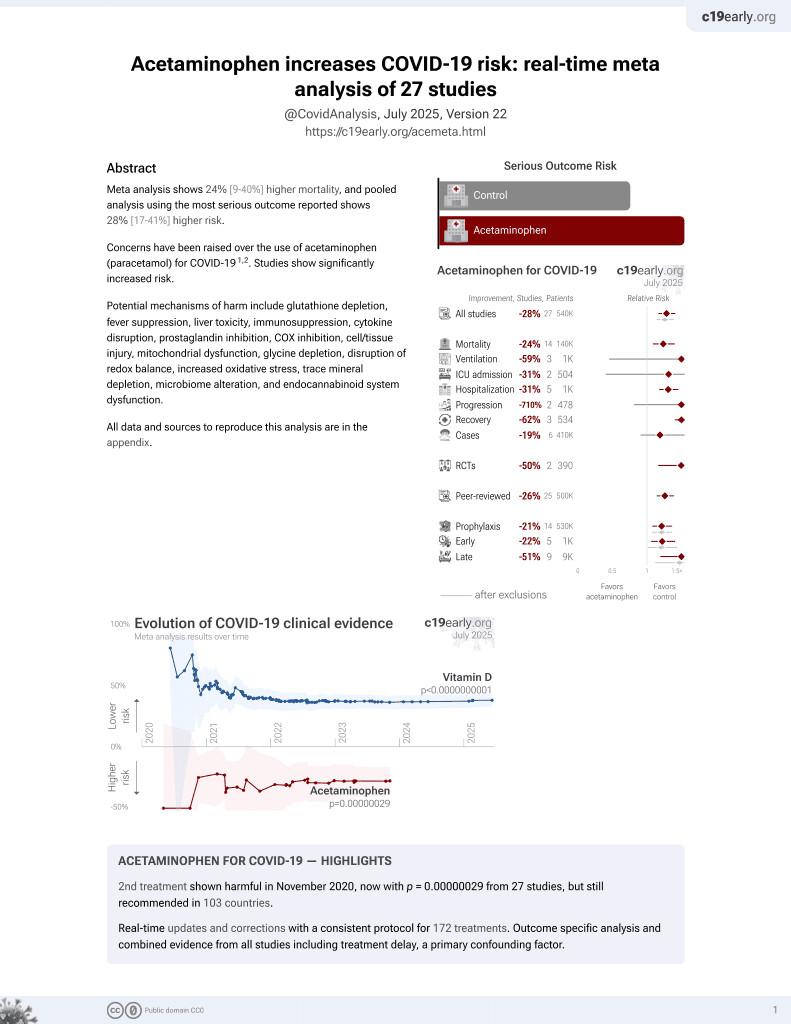
2nd treatment shown to increase risk in
November 2020, now with p = 0.00000029 from 27 studies, but still recommended in 103 countries.
6,400+ studies for
210+ treatments. c19early.org
|
Prospective study of 2,646 ICU patients ≥70 years old, showing no significant difference in mortality with acetaminophen use in the 10 days prior to ICU admission.
Acetaminophen is also known as paracetamol, Tylenol, Panadol, Calpol, Tempra, Calprofen, Doliprane, Efferalgan, Grippostad C, Dolo, Acamol, Fevadol, Crocin, and Perfalgan.
|
risk of death, 12.0% lower, OR 0.88, p = 0.20, treatment 1,166, control 1,480, adjusted per study, multivariable, day 90, RR approximated with OR.
|
|
risk of death, 14.0% lower, OR 0.86, p = 0.20, treatment 1,166, control 1,480, adjusted per study, multivariable, day 30, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Baldia et al., 27 Dec 2022, prospective, multiple countries, peer-reviewed, median age 75.0, 26 authors, trial NCT04321265 (history).
Contact: christian.jung@med.uni-duesseldorf.de (corresponding author).
The association of prior paracetamol intake with outcome of very old intensive care patients with COVID-19: results from an international prospective multicentre trial
BMC Geriatrics, doi:10.1186/s12877-022-03709-w
Background: In the early COVID-19 pandemic concerns about the correct choice of analgesics in patients with COVID-19 were raised. Little data was available on potential usefulness or harmfulness of prescription free analgesics, such as paracetamol. This international multicentre study addresses that lack of evidence regarding the usefulness or potential harm of paracetamol intake prior to ICU admission in a setting of COVID-19 disease within a large, prospectively enrolled cohort of critically ill and frail intensive care unit (ICU) patients.
Methods: This prospective international observation study (The COVIP study) recruited ICU patients ≥ 70 years admitted with COVID-19. Data on Sequential Organ Failure Assessment (SOFA) score, prior paracetamol intake within 10 days before admission, ICU therapy, limitations of care and survival during the ICU stay, at 30 days, and 3 months. Paracetamol intake was analysed for associations with ICU-, 30-day-and 3-month-mortality using Kaplan Meier analysis. Furthermore, sensitivity analyses were used to stratify 30-day-mortality in subgroups for patient-specific characteristics using logistic regression. Results: 44% of the 2,646 patients with data recorded regarding paracetamol intake within 10 days prior to ICU admission took paracetamol. There was no difference in age between patients with and without paracetamol intake. Patients taking paracetamol suffered from more co-morbidities, namely diabetes mellitus (43% versus 34%, p < 0.001), arterial hypertension (70% versus 65%, p = 0.006) and had a higher score on Clinical Frailty Scale (CFS; IQR 2-5 versus IQR 2-4, p < 0.001). Patients under prior paracetamol treatment were less often subjected to intubation and vasopressor use, compared to patients without paracetamol intake (65 versus 71%, p < 0.001; 63 versus 69%, p = 0.007). Paracetamol intake was not associated with ICU-, 30-day-and 3-month-mortality, remaining true after multivariate adjusted analysis.
Elisabeth-Krankenhaus Essen Essen
Declarations Ethics approval and consent to participate The primary competent ethics committee was the Ethics Committee of the University of Duesseldorf, Germany (application number 2020-892). Each participating center received a copy of the study protocol. Institutional research ethic board approval was obtained from each study site and was mandatory for study participation. Due to the observational nature of the study, participation in this study did not impact medical procedures, which were all executed in accordance with the relevant medical guidelines and regulations. No additional examinations, e.g., sampling and storage of biomaterials, such as blood or CT-scans and X-rays, were performed. The study was planned in adherence to the European Union General Data Privacy Regulation (GDPR) directive, which is implemented in most participating countries. Deceased patients were included within strict consideration of local requirements set up by the local ethics committees. However, in a few countries, recruitment was possible without informed consent in accordance with the respective local ethics committee (see above).
Consent for publication The manuscript does not contain any individual person's data in any form.
Competing interests The authors declare that they have no competing interests. JCS reports
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional..
References
Bruno, Wernly, Kelm, Boumendil, Morandi et al., Management and outcomes in critically ill nonagenarian versus octogenarian patients, BMC Geriatr
Bubar, Reinholt, Kissler, Lipsitch, Cobey et al., Model-informed COVID-19 vaccine prioritization strategies by age and serostatus, Science
Cabbab, Manalo, Anti-inflammatory drugs and the renin-angiotensin-aldosterone system: Current knowledge and potential effects on early SARS-CoV-2 infection, Virus Res
Chandan, Zemedikun, Thayakaran, Byne, Dhalla et al., Nonsteroidal Antiinflammatory Drugs and Susceptibility to COVID-19, Arthritis Rheumatol
Chandrasekharan, Dai, Roos, Evanson, Tomsik et al., COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression, Proc Natl Acad Sci U S A
Crighton, Mccann, Todd, Brown, Safe use of paracetamol and high-dose NSAID analgesia in dentistry during the COVID-19 pandemic, Br Dent J
Day, Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists, BMJ
Drake, Fairfield, Pius, Knight, Norman et al., Non-steroidal antiinflammatory drug use and outcomes of COVID-19 in the ISARIC Clinical Characterisation Protocol UK cohort: a matched, prospective cohort study, Lancet Rheumatol
Dwyer, Jayasekera, Nicoll, Analgesia for the cirrhotic patient: a literature review and recommendations, J Gastroenterol Hepatol
Flaatten, Jung, Beil, Guidet, The impact of end-of-life care on ICU outcome, Intensive Care Med
Flaatten, Lange, Morandi, Andersen, Artigas et al., The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (>/= 80 years), Intensive Care Med
Guidet, De Lange, Boumendil, Leaver, Watson et al., The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study, Intensive Care Med
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform
Hewitt, Carter, Vilches-Moraga, Quinn, Braude et al., The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study, Lancet Public Health
Hoffmann, Kleine-Weber, Schroeder, Kruger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell
Iraqi, Rossignol, Angioi, Fay, Nuee et al., Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study, Circulation
Jung, Bruno, Wernly, Joannidis, Oeyen et al., Inhibitors of the renin-angiotensin-aldosterone system and COVID-19 in critically ill elderly patients, Eur Heart J Cardiovasc Pharmacother
Jung, Flaatten, Fjolner, Bruno, Wernly et al., The impact of frailty on survival in elderly intensive care patients with COVID-19: the study, Crit Care
Little, Non-steroidal anti-inflammatory drugs and covid-19, BMJ
Micallef, Soeiro, Ap, French Society of Pharmacology T: Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection, Therapie
Muscedere, Waters, Varambally, Bagshaw, Boyd et al., The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis, Intensive Care Med
Nachtigall, Lenga, Jozwiak, Thurmann, Meier-Hellmann et al., Clinical course and factors associated with outcomes among 1904 patients hospitalized with COVID-19 in Germany: an observational study, Clin Microbiol Infect
Park, Lee, You, Kim, Yang, Non-steroidal anti-inflammatory agent use may not be associated with mortality of coronavirus disease 19, Sci Rep
Pharma, Pfizer, Medica, Abbott, Lilly et al., Teleflex Medical
Rinott, Kozer, Shapira, Bar-Haim, Youngster, Ibuprofen use and clinical outcomes in COVID-19 patients, Clin Microbiol Infect
Robb, Goepp, Rossi, Yao, Non-steroidal anti-inflammatory drugs, prostaglandins, and COVID-19, Br J Pharmacol
Sharma, Mehta, Paracetamol: mechanisms and updates, Contin Educ Anaesth Crit Care Pain
Smorenberg, Peters, Van Daele, Nossent, Muller, How does SARS-CoV-2 targets the elderly patients? A review on potential mechanisms increasing disease severity, Eur J Intern Med
Voiriot, Philippot, Elabbadi, Elbim, Chalumeau et al., Risks Related to the Use of Non-Steroidal Anti-Inflammatory Drugs in Community-Acquired Pneumonia in Adult and Pediatric Patients, J Clin Med
Von Philipsborn, Biallas, Burns, Drees, Geffert et al., Adverse effects of non-steroidal anti-inflammatory drugs in patients with viral respiratory infections: rapid systematic review, BMJ Open
Williamson, Walker, Bhaskaran, Bacon, Bates et al., Factors associated with COVID-19-related death using OpenSAFELY, Nature
Wu, Mcgoogan, Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention, JAMA
DOI record:
{
"DOI": "10.1186/s12877-022-03709-w",
"ISSN": [
"1471-2318"
],
"URL": "http://dx.doi.org/10.1186/s12877-022-03709-w",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>In the early COVID-19 pandemic concerns about the correct choice of analgesics in patients with COVID-19 were raised. Little data was available on potential usefulness or harmfulness of prescription free analgesics, such as paracetamol. This international multicentre study addresses that lack of evidence regarding the usefulness or potential harm of paracetamol intake prior to ICU admission in a setting of COVID-19 disease within a large, prospectively enrolled cohort of critically ill and frail intensive care unit (ICU) patients.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This prospective international observation study (The COVIP study) recruited ICU patients ≥ 70 years admitted with COVID-19. Data on Sequential Organ Failure Assessment (SOFA) score, prior paracetamol intake within 10 days before admission, ICU therapy, limitations of care and survival during the ICU stay, at 30 days, and 3 months. Paracetamol intake was analysed for associations with ICU-, 30-day- and 3-month-mortality using Kaplan Meier analysis. Furthermore, sensitivity analyses were used to stratify 30-day-mortality in subgroups for patient-specific characteristics using logistic regression.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>44% of the 2,646 patients with data recorded regarding paracetamol intake within 10 days prior to ICU admission took paracetamol. There was no difference in age between patients with and without paracetamol intake. Patients taking paracetamol suffered from more co-morbidities, namely diabetes mellitus (43% versus 34%, <jats:italic>p</jats:italic> < 0.001), arterial hypertension (70% versus 65%, <jats:italic>p</jats:italic> = 0.006) and had a higher score on Clinical Frailty Scale (CFS; IQR 2–5 versus IQR 2–4, <jats:italic>p</jats:italic> < 0.001). Patients under prior paracetamol treatment were less often subjected to intubation and vasopressor use, compared to patients without paracetamol intake (65 versus 71%, <jats:italic>p</jats:italic> < 0.001; 63 versus 69%, <jats:italic>p</jats:italic> = 0.007). Paracetamol intake was not associated with ICU-, 30-day- and 3-month-mortality, remaining true after multivariate adjusted analysis.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>Paracetamol intake prior to ICU admission was not associated with short-term and 3-month mortality in old, critically ill intensive care patients suffering from COVID-19.</jats:p>\n <jats:p>Trial registration.</jats:p>\n <jats:p>This prospective international multicentre study was registered on ClinicalTrials.gov with the identifier “NCT04321265” on March 25, 2020.\n</jats:p>\n </jats:sec>",
"alternative-id": [
"3709"
],
"article-number": "1000",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "14 January 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "19 December 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "27 December 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The primary competent ethics committee was the Ethics Committee of the University of Duesseldorf, Germany (application number 2020–892). Each participating center received a copy of the study protocol. Institutional research ethic board approval was obtained from each study site and was mandatory for study participation.Due to the observational nature of the study, participation in this study did not impact medical procedures, which were all executed in accordance with the relevant medical guidelines and regulations. No additional examinations, e.g., sampling and storage of biomaterials, such as blood or CT-scans and X-rays, were performed.The study was planned in adherence to the European Union General Data Privacy Regulation (GDPR) directive, which is implemented in most participating countries. Deceased patients were included within strict consideration of local requirements set up by the local ethics committees. However, in a few countries, recruitment was possible without informed consent in accordance with the respective local ethics committee (see above)."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The manuscript does not contain any individual person’s data in any form."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare that they have no competing interests. JCS reports grants (full departmental disclosure) from Orion Pharma, Abbott Nutrition International, B. Braun Medical AG, CSEM AG, Edwards Lifesciences Services GmbH, Kenta Biotech Ltd, Maquet Critical Care AB, Omnicare Clinical Research AG, Nestle, Pierre Fabre Pharma AG, Pfizer, Bard Medica S.A., Abbott AG, Anandic Medical Systems, Pan Gas AG Healthcare, Bracco, Hamilton Medical AG, Fresenius Kabi, Getinge Group Maquet AG, Dräger AG, Teleflex Medical GmbH, Glaxo Smith Kline, Merck Sharp and Dohme AG, Eli Lilly and Company, Baxter, Astellas, Astra Zeneca, CSL Behring, Novartis, Covidien, Philips Medical, Phagenesis Ltd, Prolong Pharmaceuticals and Nycomed outside the submitted work. The money went into departmental funds. No personal financial gain applied."
}
],
"author": [
{
"affiliation": [],
"family": "Baldia",
"given": "Philipp Heinrich",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wernly",
"given": "Bernhard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flaatten",
"given": "Hans",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fjølner",
"given": "Jesper",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Artigas",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pinto",
"given": "Bernardo Bollen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schefold",
"given": "Joerg C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kelm",
"given": "Malte",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beil",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bruno",
"given": "Raphael Romano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Binnebößel",
"given": "Stephan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wolff",
"given": "Georg",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Erkens",
"given": "Ralf",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sigal",
"given": "Sviri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Heerden",
"given": "Peter Vernon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Szczeklik",
"given": "Wojciech",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Elhadi",
"given": "Muhammed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Joannidis",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oeyen",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marsh",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andersen",
"given": "Finn H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moreno",
"given": "Rui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leaver",
"given": "Susannah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Lange",
"given": "Dylan W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guidet",
"given": "Bertrand",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jung",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eller",
"given": "Philipp",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Joannidis",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mesotten",
"given": "Dieter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reper",
"given": "Pascal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Swinnen",
"given": "Walter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Serck",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DEWAELE",
"given": "ELISABETH",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brix",
"given": "Helene",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brushoej",
"given": "Jens",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Pritpal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nedergaard",
"given": "Helene Korvenius",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balleby",
"given": "Ida Riise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bundesen",
"given": "Camilla",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hansen",
"given": "Maria Aagaard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Uhrenholt",
"given": "Stine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bundgaard",
"given": "Helle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Innes",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gooch",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cagova",
"given": "Lenka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Potter",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reay",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davey",
"given": "Miriam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abusayed",
"given": "Mohammed Abdelshafy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Humphreys",
"given": "Sally",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galbois",
"given": "Arnaud",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Charron",
"given": "Cyril",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berlemont",
"given": "Caroline Hauw",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Besch",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rigaud",
"given": "Jean-Philippe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maizel",
"given": "Julien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Djibré",
"given": "Michel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burtin",
"given": "Philippe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcon",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nseir",
"given": "Saad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valette",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alexandru",
"given": "Nica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marin",
"given": "Nathalie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vaissiere",
"given": "Marie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "PLANTEFEVE",
"given": "Gaëtan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vanderlinden",
"given": "Thierry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jurcisin",
"given": "Igor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Megarbane",
"given": "Buno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chousterman",
"given": "Benjamin Glenn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dépret",
"given": "François",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garnier",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Besset",
"given": "Sebastien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oziel",
"given": "Johanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferre",
"given": "Alexis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dauger",
"given": "Stéphane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dumas",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goncalves",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vettoretti",
"given": "Lucie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thevenin",
"given": "Didier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schaller",
"given": "Stefan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schaller",
"given": "Stefan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kurt",
"given": "Muhammed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Faltlhauser",
"given": "Andreas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schaller",
"given": "Stefan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Milovanovic",
"given": "Milena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lutz",
"given": "Matthias",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shala",
"given": "Gonxhe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haake",
"given": "Hendrik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Randerath",
"given": "Winfried",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kunstein",
"given": "Anselm",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meybohm",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schaller",
"given": "Stefan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Steiner",
"given": "Stephan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barth",
"given": "Eberhard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poerner",
"given": "Tudor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simon",
"given": "Philipp",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lorenz",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dindane",
"given": "Zouhir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kuhn",
"given": "Karl Friedrich",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Welte",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Voigt",
"given": "Ingo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kabitz",
"given": "Hans-Joachim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wollborn",
"given": "Jakob",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goebel",
"given": "Ulrich",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stoll",
"given": "Sandra Emily",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kindgen-Milles",
"given": "Detlef",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dubler",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jung",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fuest",
"given": "Kristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schuster",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Papadogoulas",
"given": "Antonios",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mulita",
"given": "Francesk",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rovina",
"given": "Nikoletta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aidoni",
"given": "Zoi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "CHRISANTHOPOULOU",
"given": "EVANGELIA",
"sequence": "additional"
},
{
"affiliation": [],
"family": "KONDILI",
"given": "EUMORFIA",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andrianopoulos",
"given": "Ioannis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Groenendijk",
"given": "Martijn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evers",
"given": "Mirjam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evers",
"given": "Mirjam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Lelyveld-Haas",
"given": "Lenneke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meynaar",
"given": "Iwan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cornet",
"given": "Alexander Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zegers",
"given": "Marieke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dieperink",
"given": "Willem",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Lange",
"given": "Dylan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dormans",
"given": "Tom",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hahn",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sjøbøe",
"given": "Britt",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Strietzel",
"given": "Hans Frank",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Olasveengen",
"given": "Theresa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romundstad",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kluzik",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zatorski",
"given": "Paweł",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Drygalski",
"given": "Tomasz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Klimkiewicz",
"given": "Jakub",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Solek-pastuszka",
"given": "Joanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Onichimowski",
"given": "Dariusz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Czuczwar",
"given": "Miroslaw",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gawda",
"given": "Ryszard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stefaniak",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stefanska-Wronka",
"given": "Karina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zabul",
"given": "Ewa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oliveira",
"given": "Ana Isabel Pinho",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Assis",
"given": "Rui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Lurdes Campos Santos",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santos",
"given": "Henrique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cardoso",
"given": "Filipe Sousa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gordinho",
"given": "André",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Banzo",
"given": "MJosé Arche",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zalba-Etayo",
"given": "Begoña",
"sequence": "additional"
},
{
"affiliation": [],
"family": "CUBERO",
"given": "PATRICIA JIMENO",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Priego",
"given": "Jesús",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gomà",
"given": "Gemma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tomasa-Irriguible",
"given": "Teresa Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sancho",
"given": "Susana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferreira",
"given": "Aida Fernández",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vázquez",
"given": "Eric Mayor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mira",
"given": "Ángela Prado",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ibarz",
"given": "Mercedes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iglesias",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arias-Rivera",
"given": "Susana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frutos-Vivar",
"given": "Fernando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lopez-Cuenca",
"given": "Sonia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aldecoa",
"given": "Cesar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perez-Torres",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Canas-Perez",
"given": "Isabel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tamayo-Lomas",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Diaz-Rodriguez",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Gopegui",
"given": "Pablo Ruiz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ben-Hamouda",
"given": "Nawfel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roberti",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fleury",
"given": "Yvan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abidi",
"given": "Nour",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dullenkopf",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pugh",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smuts",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"name": "COVIP study group",
"sequence": "additional"
}
],
"container-title": "BMC Geriatrics",
"container-title-short": "BMC Geriatr",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
12,
27
]
],
"date-time": "2022-12-27T23:02:26Z",
"timestamp": 1672182146000
},
"deposited": {
"date-parts": [
[
2022,
12,
27
]
],
"date-time": "2022-12-27T23:08:25Z",
"timestamp": 1672182505000
},
"funder": [
{
"DOI": "10.13039/501100009400",
"award": [
"2020-21",
"2018-32"
],
"doi-asserted-by": "publisher",
"name": "Medizinische Fakultät, Heinrich-Heine-Universität Düsseldorf"
},
{
"name": "Universitätsklinikum Düsseldorf. Anstalt öffentlichen Rechts"
}
],
"indexed": {
"date-parts": [
[
2022,
12,
28
]
],
"date-time": "2022-12-28T05:53:16Z",
"timestamp": 1672206796722
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
12,
27
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
27
]
],
"date-time": "2022-12-27T00:00:00Z",
"timestamp": 1672099200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
27
]
],
"date-time": "2022-12-27T00:00:00Z",
"timestamp": 1672099200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12877-022-03709-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12877-022-03709-w/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12877-022-03709-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2022,
12,
27
]
]
},
"published-online": {
"date-parts": [
[
2022,
12,
27
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.ejim.2020.11.024",
"author": "A Smorenberg",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Eur J Intern Med",
"key": "3709_CR1",
"unstructured": "Smorenberg A, Peters EJ, van Daele P, Nossent EJ, Muller M. How does SARS-CoV-2 targets the elderly patients? A review on potential mechanisms increasing disease severity. Eur J Intern Med. 2021;83:1–5.",
"volume": "83",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.2648",
"author": "Z Wu",
"doi-asserted-by": "publisher",
"first-page": "1239",
"issue": "13",
"journal-title": "JAMA",
"key": "3709_CR2",
"unstructured": "Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–42.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/j.cmi.2020.08.011",
"author": "I Nachtigall",
"doi-asserted-by": "publisher",
"first-page": "1663",
"issue": "12",
"journal-title": "Clin Microbiol Infect",
"key": "3709_CR3",
"unstructured": "Nachtigall I, Lenga P, Jozwiak K, Thurmann P, Meier-Hellmann A, Kuhlen R, Brederlau J, Bauer T, Tebbenjohanns J, Schwegmann K, et al. Clinical course and factors associated with outcomes among 1904 patients hospitalized with COVID-19 in Germany: an observational study. Clin Microbiol Infect. 2020;26(12):1663–9.",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2521-4",
"author": "EJ Williamson",
"doi-asserted-by": "publisher",
"first-page": "430",
"issue": "7821",
"journal-title": "Nature",
"key": "3709_CR4",
"unstructured": "Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–6.",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1186/s13054-021-03551-3",
"author": "C Jung",
"doi-asserted-by": "publisher",
"first-page": "149",
"issue": "1",
"journal-title": "Crit Care",
"key": "3709_CR5",
"unstructured": "Jung C, Flaatten H, Fjolner J, Bruno RR, Wernly B, Artigas A, Bollen Pinto B, Schefold JC, Wolff G, Kelm M, et al. The impact of frailty on survival in elderly intensive care patients with COVID-19: the COVIP study. Crit Care. 2021;25(1):149.",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1007/s00134-017-4867-0",
"author": "J Muscedere",
"doi-asserted-by": "publisher",
"first-page": "1105",
"issue": "8",
"journal-title": "Intensive Care Med",
"key": "3709_CR6",
"unstructured": "Muscedere J, Waters B, Varambally A, Bagshaw SM, Boyd JG, Maslove D, Sibley S, Rockwood K. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017;43(8):1105–22.",
"volume": "43",
"year": "2017"
},
{
"DOI": "10.1016/S2468-2667(20)30146-8",
"author": "J Hewitt",
"doi-asserted-by": "publisher",
"first-page": "e444",
"issue": "8",
"journal-title": "Lancet Public Health",
"key": "3709_CR7",
"unstructured": "Hewitt J, Carter B, Vilches-Moraga A, Quinn TJ, Braude P, Verduri A, Pearce L, Stechman M, Short R, Price A, et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5(8):e444–51.",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1126/science.abe6959",
"author": "KM Bubar",
"doi-asserted-by": "publisher",
"first-page": "916",
"issue": "6532",
"journal-title": "Science",
"key": "3709_CR8",
"unstructured": "Bubar KM, Reinholt K, Kissler SM, Lipsitch M, Cobey S, Grad YH, Larremore DB. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science. 2021;371(6532):916–21.",
"volume": "371",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m1086",
"author": "M Day",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "3709_CR9",
"unstructured": "Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368: m1086.",
"volume": "368",
"year": "2020"
},
{
"key": "3709_CR10",
"unstructured": "Anti-inflammatoires non stéroïdiens (AINS) et complications infectieuses graves [https://ansm.sante.fr/actualites/anti-inflammatoires-non-steroidiens-ains-et-complications-infectieuses-graves#_ftn1]"
},
{
"DOI": "10.1016/j.therap.2020.05.003",
"author": "J Micallef",
"doi-asserted-by": "publisher",
"first-page": "355",
"issue": "4",
"journal-title": "Therapie",
"key": "3709_CR11",
"unstructured": "Micallef J, Soeiro T, Jonville-Bera AP. French Society of Pharmacology T: Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection. Therapie. 2020;75(4):355–62.",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1111/bph.15206",
"author": "CT Robb",
"doi-asserted-by": "publisher",
"first-page": "4899",
"issue": "21",
"journal-title": "Br J Pharmacol",
"key": "3709_CR12",
"unstructured": "Robb CT, Goepp M, Rossi AG, Yao C. Non-steroidal anti-inflammatory drugs, prostaglandins, and COVID-19. Br J Pharmacol. 2020;177(21):4899–920.",
"volume": "177",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(21)00104-1",
"author": "TM Drake",
"doi-asserted-by": "publisher",
"first-page": "e498",
"issue": "7",
"journal-title": "Lancet Rheumatol",
"key": "3709_CR13",
"unstructured": "Drake TM, Fairfield CJ, Pius R, Knight SR, Norman L, Girvan M, Hardwick HE, Docherty AB, Thwaites RS, Openshaw PJM, et al. Non-steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC Clinical Characterisation Protocol UK cohort: a matched, prospective cohort study. Lancet Rheumatol. 2021;3(7):e498–506.",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1007/s00134-017-4940-8",
"author": "H Flaatten",
"doi-asserted-by": "publisher",
"first-page": "1820",
"issue": "12",
"journal-title": "Intensive Care Med",
"key": "3709_CR14",
"unstructured": "Flaatten H, De Lange DW, Morandi A, Andersen FH, Artigas A, Bertolini G, Boumendil A, Cecconi M, Christensen S, Faraldi L, et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (>/= 80 years). Intensive Care Med. 2017;43(12):1820–8.",
"volume": "43",
"year": "2017"
},
{
"DOI": "10.1007/s00134-019-05853-1",
"author": "B Guidet",
"doi-asserted-by": "publisher",
"first-page": "57",
"issue": "1",
"journal-title": "Intensive Care Med",
"key": "3709_CR15",
"unstructured": "Guidet B, de Lange DW, Boumendil A, Leaver S, Watson X, Boulanger C, Szczeklik W, Artigas A, Morandi A, Andersen F, et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intensive Care Med. 2020;46(1):57–69.",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1186/s12877-021-02476-4",
"author": "RR Bruno",
"doi-asserted-by": "publisher",
"first-page": "576",
"issue": "1",
"journal-title": "BMC Geriatr",
"key": "3709_CR16",
"unstructured": "Bruno RR, Wernly B, Kelm M, Boumendil A, Morandi A, Andersen FH, Artigas A, Finazzi S, Cecconi M, Christensen S, et al. Management and outcomes in critically ill nonagenarian versus octogenarian patients. BMC Geriatr. 2021;21(1):576.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1093/ehjcvp/pvaa083",
"author": "C Jung",
"doi-asserted-by": "publisher",
"first-page": "76",
"issue": "1",
"journal-title": "Eur Heart J Cardiovasc Pharmacother",
"key": "3709_CR17",
"unstructured": "Jung C, Bruno RR, Wernly B, Joannidis M, Oeyen S, Zafeiridis T, Marsh B, Andersen FH, Moreno R, Fernandes AM, et al. Inhibitors of the renin-angiotensin-aldosterone system and COVID-19 in critically ill elderly patients. Eur Heart J Cardiovasc Pharmacother. 2021;7(1):76–7.",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1016/j.jbi.2008.08.010",
"author": "PA Harris",
"doi-asserted-by": "publisher",
"first-page": "377",
"issue": "2",
"journal-title": "J Biomed Inform",
"key": "3709_CR18",
"unstructured": "Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.",
"volume": "42",
"year": "2009"
},
{
"DOI": "10.1161/CIRCULATIONAHA.108.809194",
"author": "W Iraqi",
"doi-asserted-by": "publisher",
"first-page": "2471",
"issue": "18",
"journal-title": "Circulation",
"key": "3709_CR19",
"unstructured": "Iraqi W, Rossignol P, Angioi M, Fay R, Nuee J, Ketelslegers JM, Vincent J, Pitt B, Zannad F. Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Circulation. 2009;119(18):2471–9.",
"volume": "119",
"year": "2009"
},
{
"DOI": "10.1136/bmj.m1185",
"author": "P Little",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "3709_CR20",
"unstructured": "Little P. Non-steroidal anti-inflammatory drugs and covid-19. BMJ. 2020;368: m1185.",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"author": "M Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "271",
"issue": "2",
"journal-title": "Cell",
"key": "3709_CR21",
"unstructured": "Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–80 e278.",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.virusres.2020.198190",
"author": "ILN Cabbab",
"doi-asserted-by": "publisher",
"journal-title": "Virus Res",
"key": "3709_CR22",
"unstructured": "Cabbab ILN, Manalo RVM. Anti-inflammatory drugs and the renin-angiotensin-aldosterone system: Current knowledge and potential effects on early SARS-CoV-2 infection. Virus Res. 2021;291: 198190.",
"volume": "291",
"year": "2021"
},
{
"DOI": "10.3390/jcm8060786",
"author": "G Voiriot",
"doi-asserted-by": "publisher",
"first-page": "786",
"issue": "6",
"journal-title": "J Clin Med",
"key": "3709_CR23",
"unstructured": "Voiriot G, Philippot Q, Elabbadi A, Elbim C, Chalumeau M, Fartoukh M. Risks Related to the Use of Non-Steroidal Anti-Inflammatory Drugs in Community-Acquired Pneumonia in Adult and Pediatric Patients. J Clin Med. 2019;8(6):786.",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.1073/pnas.162468699",
"author": "NV Chandrasekharan",
"doi-asserted-by": "publisher",
"first-page": "13926",
"issue": "21",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "3709_CR24",
"unstructured": "Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99(21):13926–31.",
"volume": "99",
"year": "2002"
},
{
"DOI": "10.1093/bjaceaccp/mkt049",
"author": "CV Sharma",
"doi-asserted-by": "publisher",
"first-page": "153",
"issue": "4",
"journal-title": "Continuing Education in Anaesthesia, Critical Care & Pain",
"key": "3709_CR25",
"unstructured": "Sharma CV, Mehta V. Paracetamol: mechanisms and updates. Contin Educ Anaesth Crit Care Pain. 2014;14(4):153–8.",
"volume": "14",
"year": "2014"
},
{
"DOI": "10.1136/bmjopen-2020-040990",
"author": "P von Philipsborn",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "BMJ Open",
"key": "3709_CR26",
"unstructured": "von Philipsborn P, Biallas R, Burns J, Drees S, Geffert K, Movsisyan A, Pfadenhauer LM, Sell K, Strahwald B, Stratil JM, et al. Adverse effects of non-steroidal anti-inflammatory drugs in patients with viral respiratory infections: rapid systematic review. BMJ Open. 2020;10(11): e040990.",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1038/s41415-020-1784-3",
"author": "AJ Crighton",
"doi-asserted-by": "publisher",
"first-page": "15",
"issue": "1",
"journal-title": "Br Dent J",
"key": "3709_CR27",
"unstructured": "Crighton AJ, McCann CT, Todd EJ, Brown AJ. Safe use of paracetamol and high-dose NSAID analgesia in dentistry during the COVID-19 pandemic. Br Dent J. 2020;229(1):15–8.",
"volume": "229",
"year": "2020"
},
{
"DOI": "10.1111/jgh.12560",
"author": "JP Dwyer",
"doi-asserted-by": "publisher",
"first-page": "1356",
"issue": "7",
"journal-title": "J Gastroenterol Hepatol",
"key": "3709_CR28",
"unstructured": "Dwyer JP, Jayasekera C, Nicoll A. Analgesia for the cirrhotic patient: a literature review and recommendations. J Gastroenterol Hepatol. 2014;29(7):1356–60.",
"volume": "29",
"year": "2014"
},
{
"DOI": "10.1038/s41598-021-84539-5",
"author": "J Park",
"doi-asserted-by": "publisher",
"first-page": "5087",
"issue": "1",
"journal-title": "Sci Rep",
"key": "3709_CR29",
"unstructured": "Park J, Lee SH, You SC, Kim J, Yang K. Non-steroidal anti-inflammatory agent use may not be associated with mortality of coronavirus disease 19. Sci Rep. 2021;11(1):5087.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.cmi.2020.06.003",
"author": "E Rinott",
"doi-asserted-by": "publisher",
"first-page": "1259",
"issue": "9",
"journal-title": "Clin Microbiol Infect",
"key": "3709_CR30",
"unstructured": "Rinott E, Kozer E, Shapira Y, Bar-Haim A, Youngster I. Ibuprofen use and clinical outcomes in COVID-19 patients. Clin Microbiol Infect. 2020;26(9):1259-e5.",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1002/art.41593",
"author": "JS Chandan",
"doi-asserted-by": "publisher",
"first-page": "731",
"issue": "5",
"journal-title": "Arthritis Rheumatol",
"key": "3709_CR31",
"unstructured": "Chandan JS, Zemedikun DT, Thayakaran R, Byne N, Dhalla S, Acosta-Mena D, Gokhale KM, Thomas T, Sainsbury C, Subramanian A, et al. Nonsteroidal Antiinflammatory Drugs and Susceptibility to COVID-19. Arthritis Rheumatol. 2021;73(5):731–9.",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1007/s00134-021-06365-7",
"author": "H Flaatten",
"doi-asserted-by": "publisher",
"first-page": "624",
"issue": "5",
"journal-title": "Intensive Care Med",
"key": "3709_CR32",
"unstructured": "Flaatten H, deLange D, Jung C, Beil M, Guidet B. The impact of end-of-life care on ICU outcome. Intensive Care Med. 2021;47(5):624–5.",
"volume": "47",
"year": "2021"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-022-03709-w"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Geriatrics and Gerontology"
],
"subtitle": [],
"title": "The association of prior paracetamol intake with outcome of very old intensive care patients with COVID-19: results from an international prospective multicentre trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "22"
}