Effect of convalescent plasma as complementary treatment in patients with moderate COVID-19 infection
et al., Transfusion Medicine, doi:10.1111/tme.12851, Jan 2022
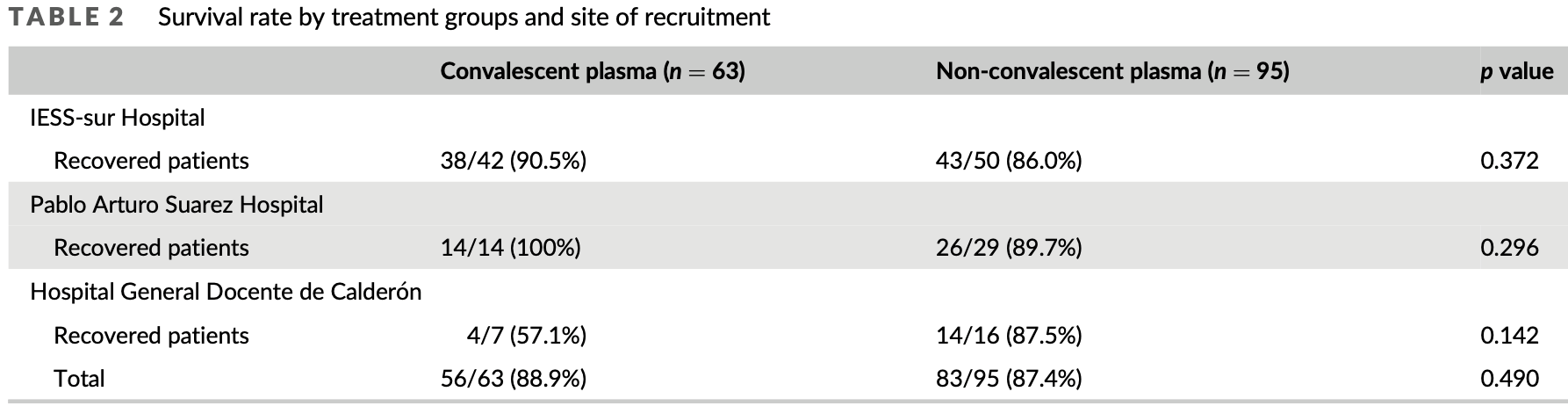
RCT 158 patients in Ecuador, showing no significant difference in mortality with convalescent plasma. Authors note indications of improved results for earlier treatment.
|
risk of death, 12.0% lower, RR 0.88, p = 1.00, treatment 7 of 63 (11.1%), control 12 of 95 (12.6%), NNT 66.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Baldeón et al., 9 Jan 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Ecuador, peer-reviewed, 17 authors, study period May 2020 - January 2021, average treatment delay 10.6 days.
Contact: mabaldeonti@uide.edu.ec.
Effect of convalescent plasma as complementary treatment in patients with moderate COVID ‐19 infection
Transfusion Medicine, doi:10.1111/tme.12851
Introduction: South America is one of the regions most affected by the COVID-19 pandemic. Specific and affordable treatments are needed to treat SARS-CoV-2 infection. Evidence regarding the use of convalescent plasma in COVID-19 patients is still limited. We compared the safety and efficacy of COVID-19-convalescent plasma administration as a complement to standard treatment in the early management of patients with moderate SARS-CoV-2 infection.
Methods: We carried out a random double blinded, placebo-controlled trial that compared standard treatment plus convalescent plasma (CP) or plus non-convalescent plasma in the management of COVID-19 patients. The main outcome was survival and secondary endpoints included: length of hospitalisation (LOH), days from treatment to discharge, time to clinical improvement or death within a 28-day period, and adverse reactions to treatment. Results: Administration of CP with antibodies against SARS-CoV-2 did not affect patient survival, RR = 1.003, 95% CI (0.3938, 2.555). These results led to terminate the RCT prematurely. However, early treatment of COVID-19 patients with CP tended to decrease the LOH while the delay in CP treatment was associated with longer hospitalisation. In addition, delay in CP treatment negatively affected the recovery of the respiratory rate. Conclusion: Use of CP for the treatment of COVID-19 patients is safe and its early use can decrease the LOH and improve respiratory function. Early administration of antibody-rich CP could contribute to decrease the negative impact of COVID-19 pandemic in patients with impaired immune response.
CONFLICT OF INTEREST The authors declare no conflict of interest.
References
Aandahl, Knutsen, Nafstad, Implementation of ISBT 128, a quality system, a standardized bar code labeling of blood products worldwide, electronic transfusion pathway: four years of experience in Norway, Transfusion
Abolghasemi, Eshghi, Cheraghali, Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study, Transfus Apher Sci
Balla, Merugu, Patel, COVID-19, modern pandemic: a systematic review from front-line health care providers' perspective, J Clin Med Res
Bennett-Guerrero, Romeiser, Talbot, Ahmed, Mamone et al., Severe acute respiratory syndrome coronavirus 2 convalescent plasma versus standard plasma in coronavirus disease 2019 infected hospitalized patients in New York: a double-blind randomized trial, Crit Care Med
Bloch, Shoham, Casadevall, Deployment of convalescent plasma for the prevention and treatment of COVID-19, J Clin Invest
Bégin, Callum, Jamula, Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial, Nat Med
Cevik, Kuppalli, Kindrachuk, Peiris, Virology, transmission, and pathogenesis of SARS-CoV-2, BMJ
Cheng, Wong, Soo, Use of convalescent plasma therapy in SARS patients in Hong Kong, Eur J Clin Microbiol Infect Dis
Gavriatopoulou, Ntanasis-Stathopoulos, Korompoki, Emerging treatment strategies for COVID-19 infection, Clin Exp Med
Gharbharan, Jordans, Geurtsvankessel, Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection, Nat Commun
Hodgins, Saad, Will the higher-income country blueprint for COVID-19 work in low-and lower middle-income countries?, Glob Heal Sci Pract
Joyner, Wright, Fairweather, Early safety indicators of COVID-19 convalescent plasma in 5000 patients, J Clin Invest
Kohmer, Westhaus, Rühl, Ciesek, Rabenau, Brief clinical evaluation of six high-throughput SARS-CoV-2 IgG antibody assays, J Clin Virol
Korley, Durkalski-Mauldin, Yeatts, Early convalescent plasma for high-risk outpatients with Covid-19, N Engl J Med
Kumar, Sah, Tripathi, Role of ACE2 receptor and the landscape of treatment options from convalescent plasma therapy to the drug repurposing in COVID-19, Mol Cell Biochem
Li, Guan, Wu, Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia, N Engl J Med
Li, Zhang, Hu, Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and lifethreatening COVID-19: a randomized clinical trial, J Am Med Assoc
Libster, Marc, Wappner, Early high-titer plasma therapy to prevent severe Covid-19 in older adults, N Engl J Med
Luke, Kilbane, Jackson, Hoffman, Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment?, Ann Intern Med
Mohtadi, Ghaysouri, Shirazi, Recovery of severely ill COVID-19 patients by intravenous immunoglobulin (IVIG) treatment: a case series, Virology
Moro, Directrices para la Obtenci on de Plasma de Donantes Convalecientes de la COVID-19 Comité Científico para la Seguridad Transfusional
Müller, Ostermann, Walker, Sensitivity of anti-SARS-CoV-2 serological assays in a high-prevalence setting, Eur J Clin Microbiol Infect Dis
O'donnell, Grinsztejn, Cummings, A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19, J Clin Invest
Piechotta, Iannizzi, Chai, Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review, Cochrane Database Syst Rev
Salazar, Christensen, Graviss, Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID-19) patients transfused early with convalescent plasma containing hightiter anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein IgG, Am J Pathol
Scott, Redmond, Tavaré, Little, Srivastava et al., Association between National Early Warning Scores in primary care and clinical outcomes: an observational study in UK primary and secondary care, Br J Gen Pract
Sette, Crotty, Adaptive immunity to SARS-CoV-2 and COVID-19, Cell
Stangel, Pul, Basic principles of intravenous immunoglobulin (IVIg) treatment, J Neurol
Stasi, Fallani, Voller, Silvestri, Treatment for COVID-19: an overview, Eur J Pharmacol
Taylor, Foo, Bruzzone, Vu Dinh, King et al., FC receptors in antibody-dependent enhancement of viral infections, Immunol Rev
Winkler, Koepsell, The use of convalescent plasma to treat emerging infectious diseases: focus on Ebola virus disease, Curr Opin Hematol
Worldometer, None, COVID live update
DOI record:
{
"DOI": "10.1111/tme.12851",
"ISSN": [
"0958-7578",
"1365-3148"
],
"URL": "http://dx.doi.org/10.1111/tme.12851",
"alternative-id": [
"10.1111/tme.12851"
],
"author": [
{
"affiliation": [
{
"name": "Escuela de Medicina, Facultad de Ciencias Médicas, de la Salud y de la Vida Universidad Internacional del Ecuador Quito Ecuador"
}
],
"family": "Baldeón",
"given": "Manuel E.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Escuela de Medicina, Colegio de Ciencias de la Salud Universidad San Francisco de Quito Quito Ecuador"
},
{
"name": "Ministerio de Salud Pública, Coordinación Zonal 9 Hospital General Docente de Calderón Quito Ecuador"
}
],
"family": "Maldonado",
"given": "Augusto",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Instituto Ecuatoriano de Seguridad Social Hospital General Quito Sur ‐ IESS Quito Ecuador"
}
],
"family": "Ochoa‐Andrade",
"given": "Miguel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ministerio de Salud Pública, Coordinación Zonal 9 Hospital General Docente de Calderón Quito Ecuador"
}
],
"family": "Largo",
"given": "Carolina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hemocentro Cruz Roja Ecuatoriana Quito Ecuador"
}
],
"family": "Pesantez",
"given": "Mónica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hemocentro Cruz Roja Ecuatoriana Quito Ecuador"
}
],
"family": "Herdoiza",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Instituto Ecuatoriano de Seguridad Social Hospital General Quito Sur ‐ IESS Quito Ecuador"
}
],
"family": "Granja",
"given": "Gerardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Instituto Ecuatoriano de Seguridad Social Hospital General Quito Sur ‐ IESS Quito Ecuador"
}
],
"family": "Bonifaz",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Instituto Ecuatoriano de Seguridad Social Hospital General Quito Sur ‐ IESS Quito Ecuador"
}
],
"family": "Espejo",
"given": "Hugo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Instituto Ecuatoriano de Seguridad Social Hospital General Quito Sur ‐ IESS Quito Ecuador"
}
],
"family": "Mora",
"given": "Francisco",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ministerio de Salud Pública, Coordinación Zonal 9 Hospital General Docente de Calderón Quito Ecuador"
}
],
"family": "Abril‐López",
"given": "Patricio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ministerio de Salud Pública, Coordinación Zonal 9 Hospital Pablo Arturo Suarez Quito Ecuador"
}
],
"family": "Armijo",
"given": "Lady Karen Robles",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ministerio de Salud Pública, Coordinación Zonal 9 Hospital Pablo Arturo Suarez Quito Ecuador"
}
],
"family": "Pacheco",
"given": "Verónica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ministerio de Salud Pública, Coordinación Zonal 9 Hospital Pablo Arturo Suarez Quito Ecuador"
}
],
"family": "Salazar",
"given": "Rafael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ministerio de Salud Pública, Coordinación Zonal 9 Hospital Pablo Arturo Suarez Quito Ecuador"
}
],
"family": "Reinthaller",
"given": "Steffy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Facultad de Ciencias de la Salud Eugenio Espejo Universidad UTE Quito Ecuador"
}
],
"family": "Zertuche",
"given": "Federico",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Escuela de Medicina, Facultad de Ciencias Médicas, de la Salud y de la Vida Universidad Internacional del Ecuador Quito Ecuador"
}
],
"family": "Fornasini",
"given": "Marco",
"sequence": "additional"
}
],
"container-title": "Transfusion Medicine",
"container-title-short": "Transfusion Medicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
10
]
],
"date-time": "2022-01-10T05:20:28Z",
"timestamp": 1641792028000
},
"deposited": {
"date-parts": [
[
2022,
4,
12
]
],
"date-time": "2022-04-12T03:20:07Z",
"timestamp": 1649733607000
},
"indexed": {
"date-parts": [
[
2022,
12,
21
]
],
"date-time": "2022-12-21T02:36:09Z",
"timestamp": 1671590169473
},
"is-referenced-by-count": 6,
"issue": "2",
"issued": {
"date-parts": [
[
2022,
1,
9
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2022,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
9
]
],
"date-time": "2022-01-09T00:00:00Z",
"timestamp": 1641686400000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
9
]
],
"date-time": "2022-01-09T00:00:00Z",
"timestamp": 1641686400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/tme.12851",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/tme.12851",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/tme.12851",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "153-161",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2022,
1,
9
]
]
},
"published-online": {
"date-parts": [
[
2022,
1,
9
]
]
},
"published-print": {
"date-parts": [
[
2022,
4
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.14740/jocmr4142",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_2_1"
},
{
"DOI": "10.9745/GHSP-D-20-00217",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_3_1"
},
{
"key": "e_1_2_9_4_1",
"unstructured": "Worldometer.COVID live update: 208 008 480 cases and 4 375 020 deaths from the coronavirus.Worldometer2021.https://www.worldometers.info/coronavirus/"
},
{
"DOI": "10.1007/s10238-020-00671-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_5_1"
},
{
"DOI": "10.1016/j.ejphar.2020.173644",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_6_1"
},
{
"DOI": "10.1172/JCI138745",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_7_1"
},
{
"DOI": "10.1007/s11010-020-03924-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_8_1"
},
{
"DOI": "10.1172/JCI140200",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_9_1"
},
{
"key": "e_1_2_9_10_1",
"unstructured": "World Health Organization.Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion as an empirical treatment during.2014p.1–19.http://apps.who.int/iris/handle/10665/135591"
},
{
"key": "e_1_2_9_11_1",
"unstructured": "NIH.Convalescent plasma and immune globulins | COVID‐19 treatment guidelines.National Institute of Health.20202020.https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/blood-derived-products/convalescent-plasma/"
},
{
"key": "e_1_2_9_12_1",
"unstructured": "World Health Organization.Therapeutics and COVID‐19: living guideline2021.https://app.magicapp.org/#/guideline/nBkO1E/section/nJB6MR"
},
{
"DOI": "10.1001/jama.2020.10044",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_13_1"
},
{
"article-title": "Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review",
"author": "Piechotta V",
"first-page": "1",
"issue": "5",
"journal-title": "Cochrane Database Syst Rev",
"key": "e_1_2_9_14_1",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.1016/j.transci.2020.102875",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_15_1"
},
{
"DOI": "10.1111/j.1537-2995.2007.01340.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_16_1"
},
{
"DOI": "10.3399/bjgp20X709337",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_17_1"
},
{
"key": "e_1_2_9_18_1",
"unstructured": "MoroE.Directrices para la Obtención de Plasma de Donantes Convalecientes de la COVID‐19 Comité Científico para la Seguridad Transfusional (CCST).2020. p. 16.https://www.mscbs.gob.es/profesionales/saludPublica/medicinaTransfusional/acuerdos/docs/COVID-19_Directrices_Plasma_donantes_convalecientes.pdf"
},
{
"DOI": "10.1097/MOH.0000000000000191",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_19_1"
},
{
"DOI": "10.7326/0003-4819-145-8-200610170-00139",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_20_1"
},
{
"DOI": "10.1038/s41467-021-23469-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_21_1"
},
{
"DOI": "10.1007/s10096-004-1271-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_22_1"
},
{
"DOI": "10.1056/NEJMoa2033700",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_23_1"
},
{
"DOI": "10.1016/j.ajpath.2020.10.008",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_24_1"
},
{
"DOI": "10.1056/NEJMoa2103784",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_25_1"
},
{
"DOI": "10.1056/NEJMoa2001316",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_26_1"
},
{
"article-title": "Virology, transmission, and pathogenesis of SARS‐CoV‐2",
"author": "Cevik M",
"first-page": "1",
"journal-title": "BMJ",
"key": "e_1_2_9_27_1",
"volume": "371",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2021.01.007",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_28_1"
},
{
"DOI": "10.1007/s10096-021-04169-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_29_1"
},
{
"DOI": "10.1016/j.jcv.2020.104480",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_30_1"
},
{
"DOI": "10.1016/j.virol.2020.05.006",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_31_1"
},
{
"DOI": "10.1007/s00415-006-5003-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_32_1"
},
{
"DOI": "10.1097/CCM.0000000000005066",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_33_1"
},
{
"DOI": "10.1172/JCI150646",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_34_1"
},
{
"DOI": "10.1111/imr.12367",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_35_1"
},
{
"DOI": "10.1038/s41591-021-01488-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_36_1"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/tme.12851"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Hematology"
],
"subtitle": [],
"title": "Effect of convalescent plasma as complementary treatment in patients with moderate\n <scp>COVID</scp>\n ‐19 infection",
"type": "journal-article",
"volume": "32"
}