Treatment with Hydroxychloroquine, Azithromycin, and Combination in Patients Hospitalized with COVID-19
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.06.099, Jul 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
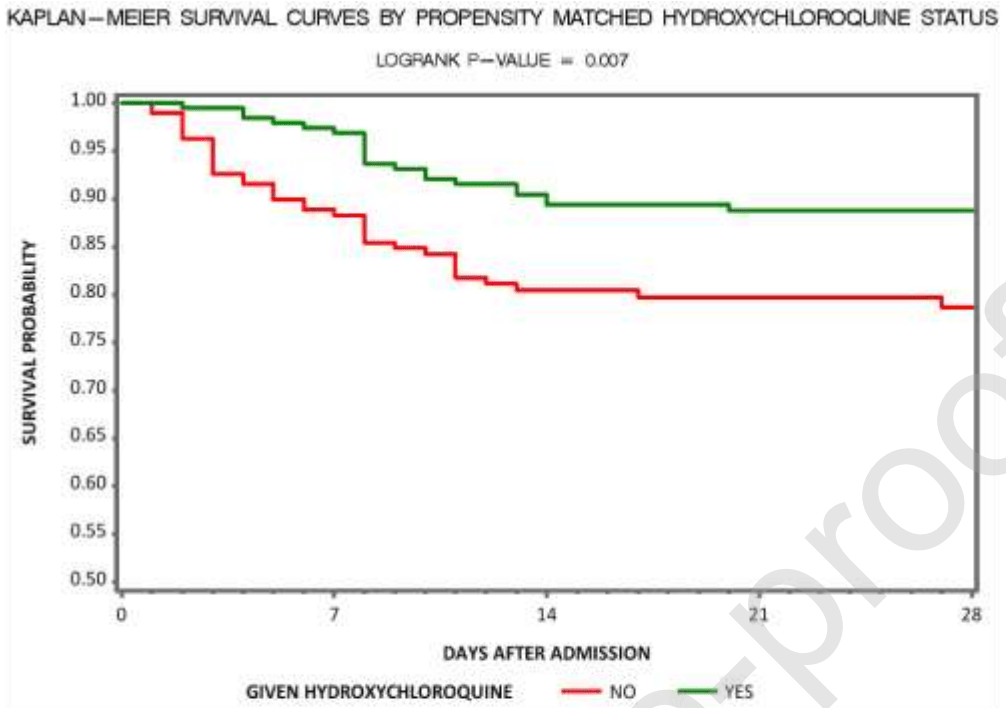
HCQ decreases mortality from 26.4% to 13.5% (HCQ) or 20.1% (HCQ+AZ). Propensity matched HCQ HR 0.487, p=0.009. Michigan 2,541 patients retrospective. Before propensity matching the HCQ group average age is 5 years younger and the percentage of male patients is 4% higher which is likely to favor the treatment and the control respectively in the before-propensity matching results.
Some reported limtiations of this study are inaccurate1. Corticosteroids were controlled for in the multivariate and propensity analyses as were age and comorbidities including cardiac disease and severity of illness. Age was an independent risk factor associated with mortality. HCQ was independently associated with decreased mortality, distinct from the steroid effect. 91% of all patients began treatment within two days of admission. HCQ was used throughout the study period, limiting time bias. Patients assigned to HCQ group had moderate and severe illness at presentation, which would favor worse outcome with HCQ.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
risk of death, 51.3% lower, HR 0.49, p = 0.009, treatment 162 of 1,202 (13.5%), control 108 of 409 (26.4%), NNT 7.7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Arshad et al., 1 Jul 2020, retrospective, USA, peer-reviewed, 12 authors.
Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19
International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.06.099
Significance: The United States is in an acceleration phase of the COVID-19 pandemic. Currently there is no known effective therapy or vaccine for treatment of SARS-CoV-2, highlighting urgency around identifying effective therapies. Objective: The purpose of this study was to evaluate the role of hydroxychloroquine therapy alone and in combination with azithromycin in hospitalized patients positive for COVID-19. Design: Multi-center retrospective observational study. Setting: The Henry Ford Health System (HFHS) in Southeast Michigan: large six hospital integrated health system; the largest of hospitals is an 802-bed quaternary academic teaching hospital in urban Detroit, Michigan. Participants: Consecutive patients hospitalized with a COVID-related admission in the health system from March 10, 2020 to May 2, 2020 were included. Only the first admission was included for patients with multiple admissions. All patients evaluated were 18 years of age and older and were treated as inpatients for at least 48 h unless expired within 24 h. Exposure: Receipt of hydroxychloroquine alone, hydroxychloroquine in combination with azithromycin, azithromycin alone, or neither. Main outcome: The primary outcome was in-hospital mortality. Results: Of 2,541 patients, with a median total hospitalization time of 6 days (IQR: 4-10 days), median age was 64 years (IQR:53-76 years), 51% male, 56% African American, with median time to follow-up of 28.5 days (IQR:3-53). Overall in-hospital mortality was 18.1% (95% CI:16.6%-19.7%); by treatment: hydroxychloroquine + azithromycin, 157/783 (20.1% [95% CI: 17.3%-23.0%]), hydroxychloroquine alone, 162/1202 (13.5% [95% CI: 11.6%-15.5%]), azithromycin alone, 33/147 (22.4% [95% CI: 16.0%-30.1%]), and neither drug, 108/409 (26.4% [95% CI: 22.2%-31.0%]). Primary cause of mortality was respiratory failure (88%); no patient had documented torsades de pointes. From Cox regression modeling, predictors of mortality were age>65 years (HR:2.6 [95% CI:1.9-3.3]), white race (HR:1.7 [95% CI:1.4-2.1]), CKD (HR:1.7 [95%CI:1.4-2.1]), reduced O2 saturation level on admission (HR:1.5 [95%CI:1.1-2.1]), and ventilator use during admission (HR: 2.2 [95%CI:1.4-3.3]). Hydroxychloroquine provided a 66% hazard ratio reduction, and hydroxychloroquine + azithromycin 71% compared to neither treatment (p < 0.001). Conclusions and relevance: In this multi-hospital assessment, when controlling for COVID-19 risk factors, treatment with hydroxychloroquine alone and in combination with azithromycin was associated with reduction in COVID-19 associated mortality. Prospective trials are needed to examine this impact.
Conflict of interest S.H. received speakers' bureau honoraria from Bayer. I.B. received speakers' bureau honoraria from Gilead, ViiV, and Jansssen, M.Z received consultation honoraria from contrafact. All others have no conflicts of interests.
Ethical approval Approval for this study was granted by the Henry Ford Hospital Institutional Review Board (#13897).
Appendix A Henry Ford COVID-19 Task Force: Varidhi Nauriyal, MD a,b , Asif Abdul Hamed, MD b , Owais Nadeem, MD b , Jennifer Swiderek, MD b , Amanda Godfrey, MD b , Jeffrey Jennings, MD b , Jayna Gardner-Gray, MD c , Adam M. Ackerman, MD d , Jonathan Lezotte, DO d , Joseph Ruhala, DO d , Raef Fadel, DO e , Amit Vahia, MD, MPH a , Smitha Gudipati, MD a , Tommy Parraga, MD a , Anita Shallal, MD a , Gina Maki, DO a , Zain Tariq, MD a , Geehan Suleyman, MD a , Nicholas Yared, MD a , Erica Herc, MD a , Johnathan Williams, MD a , Odaliz Abreu Lanfranco, MD a , Pallavi Bhargava, MD a , Katherine Reyes, MD, MPH a , Anne Chen, MD
References
Arentz, Yim, Klaff, Lokhandwala, Riedo et al., Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State, JAMA, doi:10.1001/jama.2020.4326
Cao, Wang, Wen, Liu, Jingli et al., A trial of lopinavir-ritonavir in Covid-19, N Engl J Med, doi:10.1056/nejmc2008043
Cdc, Cases in the U.S. Centers for Disease Control and Prevention
Chen, Hu, Zhang, Jiang, Han et al., Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial, MedRxiv, doi:10.1101/2020.03.22.20040758
Chen, Liu, Liu, Liu, Xu et al., A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19), J Zhejiang Univ (Med Sci)
Fda, clinical trial due to risk of heart rhythm problems
Gao, Tian, Yang, Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies, BioSci Trends, doi:10.5582/bst.2020.01047
Gautret, Lagier, Parola, Hoang, Meddeb et al., Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study, Travel Med Infect Dis, doi:10.1016/j.tmaid.2020.101663
Gautret, Lagier, Parola, Hoang, Meddeb et al., Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an openlabel non-randomized clinical trial, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.105949
Geleris, Sun, Platt, Zucker, Baldwin et al., Observational study of hydroxychloroquine in hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/nejmoa2012410
Grein, Ohmagari, Shin, Diaz, Asperges et al., Compassionate use of remdesivir for patients with severe Covid-19, N Engl J Med, doi:10.1056/nejmoa2007016
Grissom, Brown, Kuttler, Boltax, Jones et al., A modified sequential organ failure assessment score for critical care triage, Disaster Med Public Health Prep, doi:10.1001/dmp.2010.40
Guan, -Jie, Gandhi, Arons, Gandhi et al., Treatment Expert Group. Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med, doi:10.1056/NEJMoa2002032
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/s0140-6736(20)30183-5
Jung, Bobba, Su, Shariati-Sarabiet, Gladman et al., The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus, Arthritis Rheum, doi:10.1002/art.27289
Liu, Cao, Xu, Wang, Zhang et al., Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discov, doi:10.1038/s41421-020-0156-0
Magagnoli, Narendran, Pereira, Cummings, Hardin et al., Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19, doi:10.1101/2020.04.16.20065920
Mccreary Erin, Jason, Coronavirus disease 2019 treatment: a review of early and emerging options, Open Forum Infect Dis
Mccullough, Arunthamakun, Disconnect between community testing and hospitalization for SARS-CoV-2(COVID-19) infection, Baylor Univ Med Center Proc
Million, Lagier, Gautret, Colson, Fournier et al., Full-length title: early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France, Travel Med Infect Dis, doi:10.1016/j.tmaid.2020.101738
Nih, Trial of treatments for COVID-19 in hospitalized adults (DisCoVeRy) -full text view
Pagliano, Piazza, Caro, Ascione, Filippelli, Is Hydroxychloroquine a possible postexposure prophylaxis drug to limit the transmission to healthcare workers exposed to coronavirus disease 2019?, Clin Infect Dis, doi:10.1093/cid/ciaa320
References ; Andreani, Bideau, Duflot, Jardot, Rolland et al., In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect, Microb Pathog, doi:10.1016/j.micpath.2020.104228
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area, JAMA, doi:10.1001/jama.2020.6775
Rio, Malani, COVID-19-new insights on a rapidly changing epidemic, JAMA, doi:10.1001/jama.2020.3072
Rosenberg, Dufort, Udo, Wilberschied, Kumar et al., Association of treatment with hydroxychloroquine or azithromycin with inhospital mortality in patients with COVID-19 in New York State, JAMA
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic treatments for coronavirus disease 2019 (COVID-19), JAMA, doi:10.1001/jama.2020.6019
Savarino, Boelaert, Cassone, Majori, Cauda, Effects of chloroquine on viral infections: an old drug against todays diseases, Lancet Infect Dis, doi:10.1016/s1473-3099(03)00806-5
Tang, Cao, Han, Wang, Chen et al., Hydroxychloroquine in patients mainly with mild to moderate COVID-19: an open-label, randomized, controlled trial, doi:10.1101/2020.04.10.20060558
Tran, Sugamata, Hirose, Suzuki, Noguchi et al., Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process, J Antibiot, doi:10.1038/s41429-019-0204-x
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res 2020a, doi:10.1038/s41422-020-0282-0
Wang, Hu, Hu, Zhu, Liu et al., Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, doi:10.1001/jama.2020.1585
Who, Coronavirus disease (COVID-19) outbreak situation
Wu, Chen, Cai, Xia, Zhou et al., Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China, JAMA Intern Med, doi:10.1001/jamainternmed.2020.0994
Yao, Ye, Zhang, Cui, Huang et al., In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Clin Infect Dis, doi:10.1093/cid/ciaa237
Yu, Wang, Li, Hydroxychloroquine application is associated with a decreased mortality in critically ill patients with COVID-19
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/s0140-6736(20)30566-3
DOI record:
{
"DOI": "10.1016/j.ijid.2020.06.099",
"ISSN": [
"1201-9712"
],
"URL": "http://dx.doi.org/10.1016/j.ijid.2020.06.099",
"alternative-id": [
"S1201971220305348"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijid.2020.06.099"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 The Author(s). Published by Elsevier Ltd on behalf of International Society for Infectious Diseases."
}
],
"author": [
{
"affiliation": [],
"family": "Arshad",
"given": "Samia",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kilgore",
"given": "Paul",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8733-2264",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chaudhry",
"given": "Zohra S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jacobsen",
"given": "Gordon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Dee Dee",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1154-827X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Huitsing",
"given": "Kylie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brar",
"given": "Indira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alangaden",
"given": "George J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1677-3994",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ramesh",
"given": "Mayur S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3916-4021",
"affiliation": [],
"authenticated-orcid": false,
"family": "McKinnon",
"given": "John E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "O’Neill",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zervos",
"given": "Marcus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nauriyal",
"given": "Varidhi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hamed",
"given": "Asif Abdul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nadeem",
"given": "Owais",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Swiderek",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Godfrey",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jennings",
"given": "Jeffrey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gardner-Gray",
"given": "Jayna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ackerman",
"given": "Adam M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lezotte",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruhala",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fadel",
"given": "Raef",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vahia",
"given": "Amit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gudipati",
"given": "Smitha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parraga",
"given": "Tommy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shallal",
"given": "Anita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maki",
"given": "Gina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tariq",
"given": "Zain",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suleyman",
"given": "Geehan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yared",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Herc",
"given": "Erica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Williams",
"given": "Johnathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lanfranco",
"given": "Odaliz Abreu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhargava",
"given": "Pallavi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reyes",
"given": "Katherine",
"sequence": "additional"
}
],
"container-title": "International Journal of Infectious Diseases",
"container-title-short": "International Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"ijidonline.com",
"clinicalkey.jp",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.fr",
"clinicalkey.com.au",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
7,
2
]
],
"date-time": "2020-07-02T06:25:00Z",
"timestamp": 1593671100000
},
"deposited": {
"date-parts": [
[
2020,
9,
6
]
],
"date-time": "2020-09-06T14:45:57Z",
"timestamp": 1599403557000
},
"indexed": {
"date-parts": [
[
2024,
4,
1
]
],
"date-time": "2024-04-01T14:38:02Z",
"timestamp": 1711982282253
},
"is-referenced-by-count": 404,
"issued": {
"date-parts": [
[
2020,
8
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
8,
1
]
],
"date-time": "2020-08-01T00:00:00Z",
"timestamp": 1596240000000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
6,
29
]
],
"date-time": "2020-06-29T00:00:00Z",
"timestamp": 1593388800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971220305348?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971220305348?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "396-403",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
8
]
]
},
"published-print": {
"date-parts": [
[
2020,
8
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.micpath.2020.104228",
"article-title": "In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect",
"author": "Andreani",
"doi-asserted-by": "crossref",
"journal-title": "Microb Pathog",
"key": "10.1016/j.ijid.2020.06.099_bib0005",
"volume": "145",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.4326",
"article-title": "Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State",
"author": "Arentz",
"doi-asserted-by": "crossref",
"first-page": "1612",
"issue": "16",
"journal-title": "JAMA",
"key": "10.1016/j.ijid.2020.06.099_bib0010",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A trial of lopinavir–ritonavir in Covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2020.06.099_bib0015",
"year": "2020"
},
{
"author": "CDC",
"key": "10.1016/j.ijid.2020.06.099_bib0020",
"series-title": "Cases in the U.S. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)",
"year": "2020"
},
{
"article-title": "A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19)",
"author": "Chen",
"issue": "1",
"journal-title": "J Zhejiang Univ (Med Sci)",
"key": "10.1016/j.ijid.2020.06.099_bib0025",
"volume": "49",
"year": "2020"
},
{
"article-title": "Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial",
"author": "Chen",
"journal-title": "MedRxiv. 2020 preprint]",
"key": "10.1016/j.ijid.2020.06.099_bib0030",
"year": "2020"
},
{
"author": "FDA",
"key": "10.1016/j.ijid.2020.06.099_bib0035",
"series-title": "FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems",
"year": "2020"
},
{
"DOI": "10.5582/bst.2020.01047",
"article-title": "Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies",
"author": "Gao",
"doi-asserted-by": "crossref",
"first-page": "72",
"issue": "1",
"journal-title": "BioSci Trends",
"key": "10.1016/j.ijid.2020.06.099_bib0040",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"article-title": "Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial",
"author": "Gautret",
"doi-asserted-by": "crossref",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/j.ijid.2020.06.099_bib0045",
"year": "2020"
},
{
"DOI": "10.1016/j.tmaid.2020.101663",
"article-title": "Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study",
"author": "Gautret",
"doi-asserted-by": "crossref",
"journal-title": "Travel Med Infect Dis",
"key": "10.1016/j.ijid.2020.06.099_bib0050",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2012410",
"article-title": "Observational study of hydroxychloroquine in hospitalized patients with Covid-19",
"author": "Geleris",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2020.06.099_bib0055",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007016",
"article-title": "Compassionate use of remdesivir for patients with severe Covid-19",
"author": "Grein",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2020.06.099_bib0060",
"year": "2020"
},
{
"DOI": "10.1001/dmp.2010.40",
"article-title": "A modified sequential organ failure assessment score for critical care triage",
"author": "Grissom",
"doi-asserted-by": "crossref",
"first-page": "277",
"issue": "4",
"journal-title": "Disaster Med Public Health Prep",
"key": "10.1016/j.ijid.2020.06.099_bib0065",
"volume": "4",
"year": "2010"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2020.06.099_bib0070",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"issue": "10223",
"journal-title": "Lancet",
"key": "10.1016/j.ijid.2020.06.099_bib0075",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1002/art.27289",
"article-title": "The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus",
"author": "Jung",
"doi-asserted-by": "crossref",
"first-page": "863",
"issue": "3",
"journal-title": "Arthritis Rheum",
"key": "10.1016/j.ijid.2020.06.099_bib0080",
"volume": "62",
"year": "2010"
},
{
"DOI": "10.1038/s41421-020-0156-0",
"article-title": "Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro",
"author": "Liu",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Cell Discov",
"key": "10.1016/j.ijid.2020.06.099_bib0085",
"volume": "6",
"year": "2020"
},
{
"article-title": "Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19",
"author": "Magagnoli",
"journal-title": "MedRxiv. 2020 preprint]",
"key": "10.1016/j.ijid.2020.06.099_bib0090",
"year": "2020"
},
{
"article-title": "Disconnect between community testing and hospitalization for SARS-CoV-2 (COVID-19) infection",
"author": "Mc McCullough",
"journal-title": "Baylor Univ Med Center Proc",
"key": "10.1016/j.ijid.2020.06.099_bib0095",
"year": "2020"
},
{
"DOI": "10.1093/ofid/ofaa105",
"article-title": "Coronavirus disease 2019 treatment: a review of early and emerging options",
"author": "McCreary",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "Open Forum Infect Dis",
"key": "10.1016/j.ijid.2020.06.099_bib0100",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/j.tmaid.2020.101738",
"article-title": "Full-length title: early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France",
"author": "Million",
"doi-asserted-by": "crossref",
"journal-title": "Travel Med Infect Dis",
"key": "10.1016/j.ijid.2020.06.099_bib0105",
"year": "2020"
},
{
"author": "NIH",
"key": "10.1016/j.ijid.2020.06.099_bib0110",
"series-title": "Antiviral therapy",
"year": "2020"
},
{
"author": "NIH",
"key": "10.1016/j.ijid.2020.06.099_bib0115",
"series-title": "Outcomes related to COVID-19 treated with hydroxychloroquine among in-patients with symptomatic disease (ORCHID) — full text view. ClinicalTrials.gov",
"year": "2020"
},
{
"author": "NIH",
"key": "10.1016/j.ijid.2020.06.099_bib0120",
"series-title": "Trial of treatments for COVID-19 in hospitalized adults (DisCoVeRy) — full text view. ClinicalTrials.gov",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa320",
"article-title": "Is Hydroxychloroquine a possible postexposure prophylaxis drug to limit the transmission to healthcare workers exposed to coronavirus disease 2019?",
"author": "Pagliano",
"doi-asserted-by": "crossref",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ijid.2020.06.099_bib0125",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area",
"author": "Richardson",
"doi-asserted-by": "crossref",
"journal-title": "JAMA",
"key": "10.1016/j.ijid.2020.06.099_bib0130",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.3072",
"article-title": "COVID-19—new insights on a rapidly changing epidemic",
"author": "Rio",
"doi-asserted-by": "crossref",
"first-page": "1339",
"issue": "14",
"journal-title": "JAMA",
"key": "10.1016/j.ijid.2020.06.099_bib0135",
"volume": "323",
"year": "2020"
},
{
"article-title": "Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State",
"author": "Rosenberg",
"issue": "November",
"journal-title": "JAMA",
"key": "10.1016/j.ijid.2020.06.099_bib0140",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6019",
"article-title": "Pharmacologic treatments for coronavirus disease 2019 (COVID-19)",
"author": "Sanders",
"doi-asserted-by": "crossref",
"journal-title": "JAMA",
"key": "10.1016/j.ijid.2020.06.099_bib0145",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(03)00806-5",
"article-title": "Effects of chloroquine on viral infections: an old drug against todays diseases",
"author": "Savarino",
"doi-asserted-by": "crossref",
"first-page": "722",
"issue": "11",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.ijid.2020.06.099_bib0150",
"volume": "3",
"year": "2003"
},
{
"article-title": "Hydroxychloroquine in patients mainly with mild to moderate COVID-19: an open-label, randomized, controlled trial",
"author": "Tang",
"journal-title": "MedRxiv. 2020 preprint]",
"key": "10.1016/j.ijid.2020.06.099_bib0155",
"year": "2020"
},
{
"DOI": "10.1038/s41429-019-0204-x",
"article-title": "Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process",
"author": "Tran",
"doi-asserted-by": "crossref",
"first-page": "759",
"issue": "10",
"journal-title": "J Antibiot",
"key": "10.1016/j.ijid.2020.06.099_bib0160",
"volume": "72",
"year": "2019"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"issue": "3",
"journal-title": "Cell Res",
"key": "10.1016/j.ijid.2020.06.099_bib0165",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"article-title": "Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus—infected pneumonia in Wuhan, China",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1061",
"issue": "11",
"journal-title": "JAMA",
"key": "10.1016/j.ijid.2020.06.099_bib0170",
"volume": "323",
"year": "2020"
},
{
"article-title": "The cardiotoxicity of antimalarials",
"author": "WHO",
"key": "10.1016/j.ijid.2020.06.099_bib0175",
"series-title": "The cardiotoxicity of antimalarials. Malaria Policy Advisory Committee Meeting",
"year": "2017"
},
{
"author": "WHO",
"key": "10.1016/j.ijid.2020.06.099_bib0180",
"series-title": "Coronavirus disease (COVID-19) outbreak situation",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.0994",
"article-title": "Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China",
"author": "Wu",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Intern Med",
"key": "10.1016/j.ijid.2020.06.099_bib0185",
"year": "2020"
},
{
"article-title": "In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)",
"author": "Yao",
"issue": "September",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ijid.2020.06.099_bib0190",
"year": "2020"
},
{
"article-title": "Hydroxychloroquine application is associated with a decreased mortality in critically ill patients with COVID-19",
"author": "Yu",
"issue": "January",
"journal-title": "MedRxiv. 2020 preprint]",
"key": "10.1016/j.ijid.2020.06.099_bib0195",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet",
"key": "10.1016/j.ijid.2020.06.099_bib0200",
"volume": "395",
"year": "2020"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1201971220305348"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "97"
}
