A Randomized Trial of Sitagliptin and Spironolactone With Combination Therapy in Hospitalized Adults With COVID-19
et al., Journal of the Endocrine Society, doi:10.1210/jendso/bvac017, Feb 2022
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
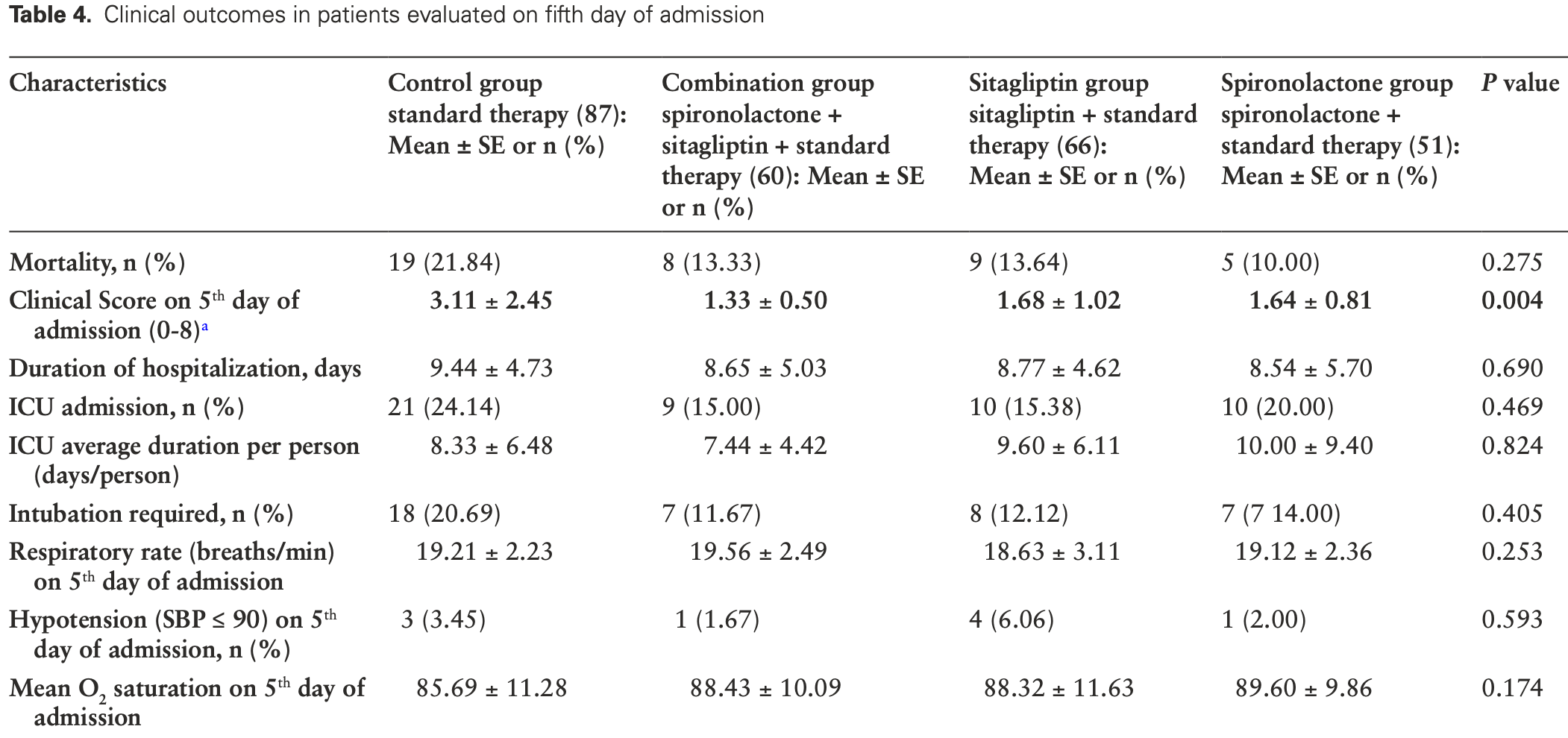
RCT including 51 spironolactone patients and 87 control patients in Iran, showing improved recovery with spironolactone, sitagliptin, and the combination of both.
|
risk of death, 55.1% lower, RR 0.45, p = 0.10, treatment 5 of 51 (9.8%), control 19 of 87 (21.8%), NNT 8.3, day 5.
|
|
risk of mechanical ventilation, 33.7% lower, RR 0.66, p = 0.36, treatment 7 of 51 (13.7%), control 18 of 87 (20.7%), NNT 14, day 5.
|
|
risk of ICU admission, 18.8% lower, RR 0.81, p = 0.67, treatment 10 of 51 (19.6%), control 21 of 87 (24.1%), NNT 22, day 5.
|
|
risk of no recovery, 47.3% lower, RR 0.53, p < 0.001, treatment mean 1.64 (±0.81) n=51, control mean 3.11 (±2.45) n=87, relative clinical score, day 5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Abbasi et al., 7 Feb 2022, Single Blind Randomized Controlled Trial, Iran, peer-reviewed, 11 authors, study period December 2020 - April 2021.
A Randomized Trial of Sitagliptin and Spironolactone With Combination Therapy in Hospitalized Adults With COVID-19
Journal of the Endocrine Society, doi:10.1210/jendso/bvac017
Context: COVID-19 may cause respiratory distress syndrome and death. Treatment of COVID-19 to prevent complications remains a priority. Objective: Our investigation sought to determine whether combination of spironolactone and sitagliptin could reduce mortality for inpatients with SARS-CoV-2 infection. Methods: This single-blind, 4-arm, prospective randomized clinical trial was conducted at Shiraz and Bushehr University of Medical Sciences hospitals between December 2020 and April 2021. We randomized hospitalized adult patients with COVID-19 pneumonia into 4 groups: control, combination therapy, sitagliptin add-on, or spironolactone add-on. The primary outcome was the clinical improvement of the patients in the hospital as measured on an 8-point numerical scale. The secondary outcomes included intubation, ICU admission, end organ damages, CT findings, and paraclinical information. Results: A total of 263 admitted patients were randomly assigned to control group (87 patients), combination group (60 patients), sitagliptin group (66 patients), and spironolactone group (50 patients). There were no significant differences in baseline characteristics, except for higher age in control group. The intervention groups, especially combination therapy, had better clinical outcomes (clinical score on fifth day of admission: 3.11 ± 2.45 for controls, 1.33 ± 0.50 for combination, 1.68 ± 1.02 for sitagliptin, and 1.64 ± 0.81 for spironolactone; P = 0.004). However, the mortality rate was lower in patients who received spironolactone (21.84% control, 13.33% combination, 13.64% sitagliptin, 10.00% spironolactone; P = 0.275). Our intervention reduced lung infiltration but not the area of involvement in lungs. Conclusion: Sitagliptin and spironolactone can potentially improve clinical outcomes of hospitalized COVID-19 patients.
Abbreviations: CT, computed tomography; ECMO, extracorporeal membrane oxygenation; PCR, polymerase chain reaction.
Financial Support This project is supported by Vice-Chancellor for Research at the Shiraz University of Medical Sciences, Bushehr University Medical Sciences, Faghihi Hospital and Shohadaye_Khalije_ Fars Hospital.
Author Contributions Kamyar Asadipooya proposed the idea, designed the study, and wrote the manuscript. Farhad Abbasi and Mohammad Ali Davarpanah helped designi the study. Reuben Adatorwovor provided statistical analysis, wrote the statistical portions, and revised the manuscript. Sepideh Sefidbakht and Farhad abbasi read the CT scan results. Yasaman Mansoori, Mehdi Hajiani, Farzan Azodi, Shayesteh Davoudi, and Farzana Rezaei collected the data. Shayan Mohammadmoradi helped with designing the study and editing the manuscript. All authors approved the final version of the manuscript.
Disclosures The authors have declared that no conflict of interest exists.
Iranian Registry of Clinical Trial Information Iranian Registry of Clinical Trial registration number: IRCT20201003048904N2; registration date: December 10, 2020.
References
Alqahtani, Abdulrahman, Almadani, Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease, Sci Rep
Christie, Henley, Mattocks, Decreases in COVID-19 cases, emergency department visits, hospital admissions, and deaths among older adults following the introduction of COVID-19 vaccine-United States, September 6, 2020, MMWR Morb Mortal Wkly Rep
Dai, Heemers, Sharifi, Androgen signaling in prostate cancer, Cold Spring Harb Perspect Med
Ding, Xu, Zhou, Long, Chest CT findings of COVID-19 pneumonia by duration of symptoms, Eur J Radiol
Donato, Park, Baker, Clinical and laboratory evaluation of patients with SARS-CoV-2 pneumonia treated with hightiter convalescent plasma, JCI Insight
Dong, Fan, Ji, Yu, Wu et al., Spironolactone alleviates diabetic nephropathy through promoting autophagy in podocytes, Int Urol Nephrol
Fukuda, Horimai, Harada, Aldosterone-induced kidney injury is mediated by NFκB activation, Clin Exp Nephrol
Gharbharan, Jordans, Geurtsvankessel, Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection, Nat Commun
Haga, Nagata, Okamura, TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds, Antiviral Res
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Ishfaq, Farooq, Goraya, Role of high resolution computed tomography chest in the diagnosis and evaluation of COVID -19 patients -A systematic review and meta-analysis, Eur J Radiol Open
Jeon, Son, Choi, Effect of Spironolactone on COVID-19 in patients with underlying liver cirrhosis: a nationwide case-control study in South Korea, Front Med
Keidar, Gamliel-Lazarovich, Kaplan, Mineralocorticoid receptor blocker increases angiotensinconverting enzyme 2 activity in congestive heart failure patients, Circ Res
Khalagi, Gharibzadeh, Khalili, Prevalence of COVID-19 in Iran: results of the first survey of the Iranian COVID-19 Serological Surveillance program, Clin Microbiol Infect
Kombe, Zahid, Mohammed, Shi, Potent molecular feature-based neutralizing monoclonal antibodies as promising therapeutics against SARS-CoV-2 infection, Front Mol Biosci
Kuba, Imai, Rao, A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury, Nat Med
Kustin, Harel, Finkel, Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals, Nat Med
Li, Liu, Zhou, Wang, On resistance to virus entry into host cells, Biophys J
Li, Zhang, Hu, Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial, JAMA
Li, Zhang, Sui, Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2, Embo J
Li, Zhang, Yang, The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike, Iscience
Libster, Marc, Wappner, Fundación INFANT-COVID-19 Group. Early high-titer plasma therapy to prevent severe covid-19 in older adults, N Engl J Med
Mahmudpour, Roozbeh, Keshavarz, Farrokhi, Nabipour, COVID-19 cytokine storm: The anger of inflammation, Cytokine
Mareev, Orlova, Plisyk, BromhexIne and Spironolactone for CoronаvirUs Infection requiring hospiTalization (BISCUIT)
Martinez, Compounds with therapeutic potential against novel respiratory 2019 coronavirus, Antimicrob Agents Chemother
Millet, Whittaker, Host cell proteases: critical determinants of coronavirus tropism and pathogenesis, Virus Res
Montastruc, Romano, Montastruc, Pharmacological characteristics of patients infected with SARS-Cov-2 admitted to Intensive Care Unit in South of France, Therapie
Monteil, Kwon, Prado, Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2, Cell
Müller-Olling, Vahlensieck, Hilger, Heterogeneity in COVID-19 convalescent plasma clinical trials, Clin Pharmacol Therapeut
Qi, Qian, Zhang, Zhang, Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses, Biochem Biophys Res Commun
Roberts, Driggs, Thorpe, Common pitfalls and recommendations for using machine learning to detect and prognosticate for COVID-19 using chest radiographs and CT scans, Nat Mach Intell
Satoh, Ishikawa, Minami, Akatsu, Nakamura, Eplerenone inhibits tumour necrosis factor alpha shedding process by tumour necrosis factor alpha converting enzyme in monocytes from patients with congestive heart failure, Heart
Shao, Xu, Yu, Pan, Chen, Dipeptidyl peptidase 4 inhibitors and their potential immune modulatory functions, Pharmacol Ther
Shi, Wang, Shao, COVID-19 infection: the perspectives on immune responses, Cell Death Differ
Solerte, 'addio, Trevisan, Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study, Diabetes Care
Tai, He, Zhang, Characterization of the receptorbinding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine, Cell Mol Immunol
Tomlins, Rhodes, Perner, Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer, Science
Vankadari, Wilce, Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26, Emerg Microbes Infect
Verdecchia, Cavallini, Spanevello, Angeli, The pivotal link between ACE2 deficiency and SARS-CoV-2 infection, Eur J Intern Med
Vilaca, Schini, Harnan, The risk of hip and nonvertebral fractures in type 1 and type 2 diabetes: a systematic review and meta-analysis update, Bone
Walls, Park, Tortorici, Wall, Mcguire et al., Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein, Cell
Wardhani, Fajar, Wulandari, Association between convalescent plasma and the risk of mortality among patients with COVID-19: a meta-analysis
Zoufaly, Poglitsch, Aberle, Human recombinant soluble ACE2 in severe COVID-19, Lancet Respir Med
DOI record:
{
"DOI": "10.1210/jendso/bvac017",
"ISSN": [
"2472-1972"
],
"URL": "http://dx.doi.org/10.1210/jendso/bvac017",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Context</jats:title>\n <jats:p>COVID-19 may cause respiratory distress syndrome and death. Treatment of COVID-19 to prevent complications remains a priority.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Objective</jats:title>\n <jats:p>Our investigation sought to determine whether combination of spironolactone and sitagliptin could reduce mortality for inpatients with SARS-CoV-2 infection.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This single-blind, 4-arm, prospective randomized clinical trial was conducted at Shiraz and Bushehr University of Medical Sciences hospitals between December 2020 and April 2021. We randomized hospitalized adult patients with COVID-19 pneumonia into 4 groups: control, combination therapy, sitagliptin add-on, or spironolactone add-on. The primary outcome was the clinical improvement of the patients in the hospital as measured on an 8-point numerical scale. The secondary outcomes included intubation, ICU admission, end organ damages, CT findings, and paraclinical information.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of 263 admitted patients were randomly assigned to control group (87 patients), combination group (60 patients), sitagliptin group (66 patients), and spironolactone group (50 patients). There were no significant differences in baseline characteristics, except for higher age in control group. The intervention groups, especially combination therapy, had better clinical outcomes (clinical score on fifth day of admission: 3.11 ± 2.45 for controls, 1.33 ± 0.50 for combination, 1.68 ± 1.02 for sitagliptin, and 1.64 ± 0.81 for spironolactone; P = 0.004). However, the mortality rate was lower in patients who received spironolactone (21.84% control, 13.33% combination, 13.64% sitagliptin, 10.00% spironolactone; P = 0.275). Our intervention reduced lung infiltration but not the area of involvement in lungs.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>Sitagliptin and spironolactone can potentially improve clinical outcomes of hospitalized COVID-19 patients.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Bushehr University of Medical Sciences, Bushehr 75179-33755, Iran"
}
],
"family": "Abbasi",
"given": "Farhad",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, University of Kentucky, Lexington, KY 40536,USA"
}
],
"family": "Adatorwovor",
"given": "Reuben",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Shiraz HIV/AIDS Research Center, Shiraz University of Medical Sciences, Shiraz 71348-14336, Iran"
}
],
"family": "Davarpanah",
"given": "Mohammad Ali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Student Research Committee, Shiraz University of Medical Sciences, Shiraz 71348-14336, Iran"
}
],
"family": "Mansoori",
"given": "Yasaman",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Student Research Committee, Shiraz University of Medical Sciences, Shiraz 71348-14336, Iran"
}
],
"family": "Hajiani",
"given": "Mehdi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine, Bushehr University of Medical Sciences, Bushehr 75179-33755, Iran"
}
],
"family": "Azodi",
"given": "Farzan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Imaging Research Center, Department of Radiology, Shiraz University of Medical Sciences, Shiraz 71348-14336, Iran"
}
],
"family": "Sefidbakht",
"given": "Sepideh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Student Research Committee, Bushehr University of Medical Sciences, Bushehr 75179-33755, Iran"
}
],
"family": "Davoudi",
"given": "Shayesteh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Student Research Committee, Bushehr University of Medical Sciences, Bushehr 75179-33755, Iran"
}
],
"family": "Rezaei",
"given": "Farzana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Saha Cardiovascular Research Center, University of Kentucky, Lexington, KY 40536, USA"
},
{
"name": "Department of Pharmacology and Nutritional Sciences, University of Kentucky, Lexington, KY 40536,USA"
}
],
"family": "Mohammadmoradi",
"given": "Shayan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4484-1971",
"affiliation": [
{
"name": "Assistant Professor of Medicine, Department of Medicine, Division of Endocrinology, Diabetes, and Metabolism, Barnstable Brown Diabetes and Obesity Center, University of Kentucky, Lexington, KY 40504, USA"
}
],
"authenticated-orcid": false,
"family": "Asadipooya",
"given": "Kamyar",
"sequence": "additional"
}
],
"container-title": [
"Journal of the Endocrine Society"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
9
]
],
"date-time": "2022-02-09T02:36:55Z",
"timestamp": 1644374215000
},
"deposited": {
"date-parts": [
[
2022,
3,
5
]
],
"date-time": "2022-03-05T15:33:26Z",
"timestamp": 1646494406000
},
"funder": [
{
"name": "Vice-Chancellor for Research at the Shiraz University of Medical Sciences"
},
{
"name": "Bushehr University Medical Sciences"
},
{
"name": "Faghihi Hospital and Shohadaye_Khalije_Fars Hospital"
}
],
"indexed": {
"date-parts": [
[
2022,
4,
5
]
],
"date-time": "2022-04-05T03:46:38Z",
"timestamp": 1649130398817
},
"is-referenced-by-count": 1,
"issn-type": [
{
"type": "electronic",
"value": "2472-1972"
}
],
"issue": "4",
"issued": {
"date-parts": [
[
2022,
2,
7
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2022,
4,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
7
]
],
"date-time": "2022-02-07T00:00:00Z",
"timestamp": 1644192000000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/jes/advance-article-pdf/doi/10.1210/jendso/bvac017/42424073/bvac017.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jes/article-pdf/6/4/bvac017/42747317/bvac017.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jes/article-pdf/6/4/bvac017/42747317/bvac017.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "80",
"original-title": [],
"prefix": "10.1210",
"published": {
"date-parts": [
[
2022,
2,
7
]
]
},
"published-online": {
"date-parts": [
[
2022,
2,
7
]
]
},
"published-other": {
"date-parts": [
[
2022,
4,
1
]
]
},
"published-print": {
"date-parts": [
[
2022,
4,
1
]
]
},
"publisher": "The Endocrine Society",
"reference": [
{
"DOI": "10.1056/NEJMoa2033700",
"article-title": "Early high-titer plasma therapy to prevent severe covid-19 in older adults",
"author": "Libster",
"doi-asserted-by": "crossref",
"first-page": "610",
"issue": "7",
"journal-title": "N Engl J Med.",
"key": "2022030515323874600_CIT0001",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1172/jci.insight.143196",
"article-title": "Clinical and laboratory evaluation of patients with SARS-CoV-2 pneumonia treated with high-titer convalescent plasma",
"author": "Donato",
"doi-asserted-by": "crossref",
"first-page": "e143196",
"issue": "6",
"journal-title": "JCI Insight.",
"key": "2022030515323874600_CIT0002",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.10044",
"article-title": "Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "460",
"issue": "5",
"journal-title": "JAMA.",
"key": "2022030515323874600_CIT0003",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1038/s41598-021-89444-5",
"article-title": "Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease",
"author": "AlQahtani",
"doi-asserted-by": "crossref",
"first-page": "9927",
"issue": "1",
"journal-title": "Sci Rep.",
"key": "2022030515323874600_CIT0004",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-23469-2",
"article-title": "Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection",
"author": "Gharbharan",
"doi-asserted-by": "crossref",
"first-page": "3189",
"issue": "1",
"journal-title": "Nat Commun.",
"key": "2022030515323874600_CIT0005",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.11.032",
"article-title": "Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein",
"author": "Walls",
"doi-asserted-by": "crossref",
"first-page": "1735",
"issue": "6",
"journal-title": "Cell.",
"key": "2022030515323874600_CIT0006",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1080/22221751.2020.1739565",
"article-title": "Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26",
"author": "Vankadari",
"doi-asserted-by": "crossref",
"first-page": "601",
"issue": "1",
"journal-title": "Emerg Microbes Infect.",
"key": "2022030515323874600_CIT0007",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.isci.2020.101160",
"article-title": "The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "101160",
"issue": "6",
"journal-title": "Iscience.",
"key": "2022030515323874600_CIT0008",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.1038/sj.emboj.7600640",
"article-title": "Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1634",
"issue": "8",
"journal-title": "Embo J.",
"key": "2022030515323874600_CIT0009",
"volume": "24",
"year": "2005"
},
{
"DOI": "10.1128/AAC.00399-20",
"article-title": "Compounds with therapeutic potential against novel respiratory 2019 coronavirus",
"author": "Martinez",
"doi-asserted-by": "crossref",
"first-page": "e00399",
"issue": "5",
"journal-title": "Antimicrob Agents Chemother.",
"key": "2022030515323874600_CIT0010",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"issue": "2",
"journal-title": "Cell.",
"key": "2022030515323874600_CIT0011",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.virusres.2014.11.021",
"article-title": "Host cell proteases: critical determinants of coronavirus tropism and pathogenesis",
"author": "Millet",
"doi-asserted-by": "crossref",
"first-page": "120",
"journal-title": "Virus Res.",
"key": "2022030515323874600_CIT0012",
"volume": "202",
"year": "2015"
},
{
"DOI": "10.1016/j.bpj.2012.03.066",
"article-title": "On resistance to virus entry into host cells",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "2230",
"issue": "9",
"journal-title": "Biophys J.",
"key": "2022030515323874600_CIT0013",
"volume": "102",
"year": "2012"
},
{
"DOI": "10.1038/nm1267",
"article-title": "A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury",
"author": "Kuba",
"doi-asserted-by": "crossref",
"first-page": "875",
"issue": "8",
"journal-title": "Nat Med.",
"key": "2022030515323874600_CIT0014",
"volume": "11",
"year": "2005"
},
{
"DOI": "10.1016/j.cyto.2020.155151",
"article-title": "COVID-19 cytokine storm: The anger of inflammation",
"author": "Mahmudpour",
"doi-asserted-by": "crossref",
"first-page": "155151",
"journal-title": "Cytokine.",
"key": "2022030515323874600_CIT0015",
"volume": "133",
"year": "2020"
},
{
"DOI": "10.1016/j.ejim.2020.04.037",
"article-title": "The pivotal link between ACE2 deficiency and SARS-CoV-2 infection",
"author": "Verdecchia",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "Eur J Intern Med.",
"key": "2022030515323874600_CIT0016",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1007/s11255-019-02074-9",
"article-title": "Spironolactone alleviates diabetic nephropathy through promoting autophagy in podocytes",
"author": "Dong",
"doi-asserted-by": "crossref",
"first-page": "755",
"issue": "4",
"journal-title": "Int Urol Nephrol.",
"key": "2022030515323874600_CIT0017",
"volume": "51",
"year": "2019"
},
{
"DOI": "10.1161/01.RES.0000187500.24964.7A",
"article-title": "Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients",
"author": "Keidar",
"doi-asserted-by": "crossref",
"first-page": "946",
"issue": "9",
"journal-title": "Circ Res.",
"key": "2022030515323874600_CIT0018",
"volume": "97",
"year": "2005"
},
{
"DOI": "10.1126/science.1117679",
"article-title": "Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer",
"author": "Tomlins",
"doi-asserted-by": "crossref",
"first-page": "644",
"issue": "5748",
"journal-title": "Science.",
"key": "2022030515323874600_CIT0019",
"volume": "310",
"year": "2005"
},
{
"DOI": "10.1101/cshperspect.a030452",
"article-title": "Androgen signaling in prostate cancer",
"author": "Dai",
"doi-asserted-by": "crossref",
"first-page": "a030452",
"issue": "(9)",
"journal-title": "Cold Spring Harb Perspect Med",
"key": "2022030515323874600_CIT0020",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1016/j.pharmthera.2020.107503",
"article-title": "Dipeptidyl peptidase 4 inhibitors and their potential immune modulatory functions",
"author": "Shao",
"doi-asserted-by": "crossref",
"first-page": "107503",
"journal-title": "Pharmacol Ther.",
"key": "2022030515323874600_CIT0021",
"volume": "209",
"year": "2020"
},
{
"DOI": "10.1016/j.bbrc.2020.03.044",
"article-title": "Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses",
"author": "Qi",
"doi-asserted-by": "crossref",
"first-page": "135",
"issue": "1",
"journal-title": "Biochem Biophys Res Commun.",
"key": "2022030515323874600_CIT0022",
"volume": "526",
"year": "2020"
},
{
"DOI": "10.1038/s41423-020-0400-4",
"article-title": "Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine",
"author": "Tai",
"doi-asserted-by": "crossref",
"first-page": "613",
"journal-title": "Cell Mol Immunol.",
"key": "2022030515323874600_CIT0023",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.2337/dc20-1521",
"article-title": "Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study",
"author": "Solerte",
"doi-asserted-by": "crossref",
"first-page": "2999",
"issue": "12",
"journal-title": "Diabetes Care.",
"key": "2022030515323874600_CIT0024",
"volume": "43",
"year": "2020"
},
{
"DOI": "10.1016/j.therap.2020.05.005",
"article-title": "Pharmacological characteristics of patients infected with SARS-Cov-2 admitted to Intensive Care Unit in South of France",
"author": "Montastruc",
"doi-asserted-by": "crossref",
"first-page": "381",
"issue": "4",
"journal-title": "Therapie.",
"key": "2022030515323874600_CIT0025",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1016/j.bone.2020.115457",
"article-title": "The risk of hip and non-vertebral fractures in type 1 and type 2 diabetes: a systematic review and meta-analysis update",
"author": "Vilaca",
"doi-asserted-by": "crossref",
"first-page": "115457",
"journal-title": "Bone.",
"key": "2022030515323874600_CIT0026",
"volume": "137",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm7023e2",
"article-title": "Decreases in COVID-19 cases, emergency department visits, hospital admissions, and deaths among older adults following the introduction of COVID-19 vaccine—United States, September 6, 2020-May 1, 2021",
"author": "Christie",
"doi-asserted-by": "crossref",
"first-page": "858",
"issue": "23",
"journal-title": "MMWR Morb Mortal Wkly Rep.",
"key": "2022030515323874600_CIT0027",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01413-7",
"article-title": "Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals",
"author": "Kustin",
"doi-asserted-by": "crossref",
"first-page": "1379",
"issue": "8",
"journal-title": "Nat Med.",
"key": "2022030515323874600_CIT0028",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1016/j.cmi.2021.06.002",
"article-title": "Prevalence of COVID-19 in Iran: results of the first survey of the Iranian COVID-19 Serological Surveillance program",
"author": "Khalagi",
"doi-asserted-by": "crossref",
"journal-title": "Clin Microbiol Infect.",
"key": "2022030515323874600_CIT0029",
"year": "2021"
},
{
"DOI": "10.12688/f1000research.36396.1",
"article-title": "Association between convalescent plasma and the risk of mortality among patients with COVID-19: a meta-analysis",
"author": "Wardhani",
"doi-asserted-by": "crossref",
"first-page": "64",
"journal-title": "F1000Res.",
"key": "2022030515323874600_CIT0030",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.3389/fmolb.2021.670815",
"article-title": "Potent molecular feature-based neutralizing monoclonal antibodies as promising therapeutics against SARS-CoV-2 infection",
"author": "Kombe Kombe",
"doi-asserted-by": "crossref",
"first-page": "670815",
"journal-title": "Front Mol Biosci.",
"key": "2022030515323874600_CIT0031",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1002/cpt.2281",
"article-title": "Heterogeneity in COVID-19 convalescent plasma clinical trials",
"author": "Müller-Olling",
"doi-asserted-by": "crossref",
"journal-title": "Clin Pharmacol Therapeut",
"key": "2022030515323874600_CIT0032",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(20)30418-5",
"article-title": "Human recombinant soluble ACE2 in severe COVID-19",
"author": "Zoufaly",
"doi-asserted-by": "crossref",
"first-page": "1154",
"issue": "11",
"journal-title": "Lancet Respir Med.",
"key": "2022030515323874600_CIT0033",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.04.004",
"article-title": "Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2",
"author": "Monteil",
"doi-asserted-by": "crossref",
"first-page": "905",
"journal-title": "Cell.",
"key": "2022030515323874600_CIT0034",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2009.12.001",
"article-title": "TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds",
"author": "Haga",
"doi-asserted-by": "crossref",
"first-page": "551",
"issue": "3",
"journal-title": "Antiviral Res.",
"key": "2022030515323874600_CIT0035",
"volume": "85",
"year": "2010"
},
{
"DOI": "10.1136/hrt.2005.071829",
"article-title": "Eplerenone inhibits tumour necrosis factor alpha shedding process by tumour necrosis factor alpha converting enzyme in monocytes from patients with congestive heart failure",
"author": "Satoh",
"doi-asserted-by": "crossref",
"first-page": "979",
"issue": "7",
"journal-title": "Heart.",
"key": "2022030515323874600_CIT0036",
"volume": "92",
"year": "2006"
},
{
"DOI": "10.1007/s10157-010-0373-1",
"article-title": "Aldosterone-induced kidney injury is mediated by NFκB activation",
"author": "Fukuda",
"doi-asserted-by": "crossref",
"first-page": "41",
"issue": "1",
"journal-title": "Clin Exp Nephrol.",
"key": "2022030515323874600_CIT0037",
"volume": "15",
"year": "2011"
},
{
"DOI": "10.3389/fmed.2021.629176",
"article-title": "Effect of Spironolactone on COVID-19 in patients with underlying liver cirrhosis: a nationwide case-control study in South Korea",
"author": "Jeon",
"doi-asserted-by": "crossref",
"first-page": "629176",
"journal-title": "Front Med (Lausanne).",
"key": "2022030515323874600_CIT0038",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.18087/cardio.2020.11.n1440",
"article-title": "[Results of Open-Label non-Randomized Comparative Clinical Trial: “BromhexIne and Spironolactone for CoronаvirUs Infection requiring hospiTalization (BISCUIT)]",
"author": "Mareev",
"doi-asserted-by": "crossref",
"first-page": "4",
"issue": "11",
"journal-title": "Kardiologiia.",
"key": "2022030515323874600_CIT0039",
"volume": "60",
"year": "2020"
},
{
"DOI": "10.1038/s41418-020-0530-3",
"article-title": "COVID-19 infection: the perspectives on immune responses",
"author": "Shi",
"doi-asserted-by": "crossref",
"first-page": "1451",
"issue": "5",
"journal-title": "Cell Death Differ.",
"key": "2022030515323874600_CIT0040",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1016/j.ejrad.2020.109009",
"article-title": "Chest CT findings of COVID-19 pneumonia by duration of symptoms",
"author": "Ding",
"doi-asserted-by": "crossref",
"first-page": "109009",
"journal-title": "Eur J Radiol.",
"key": "2022030515323874600_CIT0041",
"volume": "127",
"year": "2020"
},
{
"DOI": "10.1016/j.ejro.2021.100350",
"article-title": "Role of high resolution computed tomography chest in the diagnosis and evaluation of COVID -19 patients -A systematic review and meta-analysis",
"author": "Ishfaq",
"doi-asserted-by": "crossref",
"first-page": "100350",
"journal-title": "Eur J Radiol Open.",
"key": "2022030515323874600_CIT0042",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1038/s42256-021-00307-0",
"article-title": "Common pitfalls and recommendations for using machine learning to detect and prognosticate for COVID-19 using chest radiographs and CT scans",
"author": "Roberts",
"doi-asserted-by": "crossref",
"first-page": "199",
"journal-title": "Nat Mach Intell",
"key": "2022030515323874600_CIT0043",
"volume": "3",
"year": "2021"
}
],
"reference-count": 43,
"references-count": 43,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jes/article/doi/10.1210/jendso/bvac017/6523815"
}
},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subject": [
"Endocrinology, Diabetes and Metabolism"
],
"subtitle": [],
"title": [
"A Randomized Trial of Sitagliptin and Spironolactone With Combination Therapy in Hospitalized Adults With COVID-19"
],
"type": "journal-article",
"volume": "6"
}
