Effects of Lianhuaqingwen Capsules in adults with mild-to-moderate coronavirus disease 2019: an international, multicenter, double-blind, randomized controlled trial
et al., Virology Journal, doi:10.1186/s12985-023-02144-6, FLOSAN, ChiCTR2200056727, Nov 2023
Quercetin for COVID-19
27th treatment shown to reduce risk in
July 2021, now with p = 0.002 from 12 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
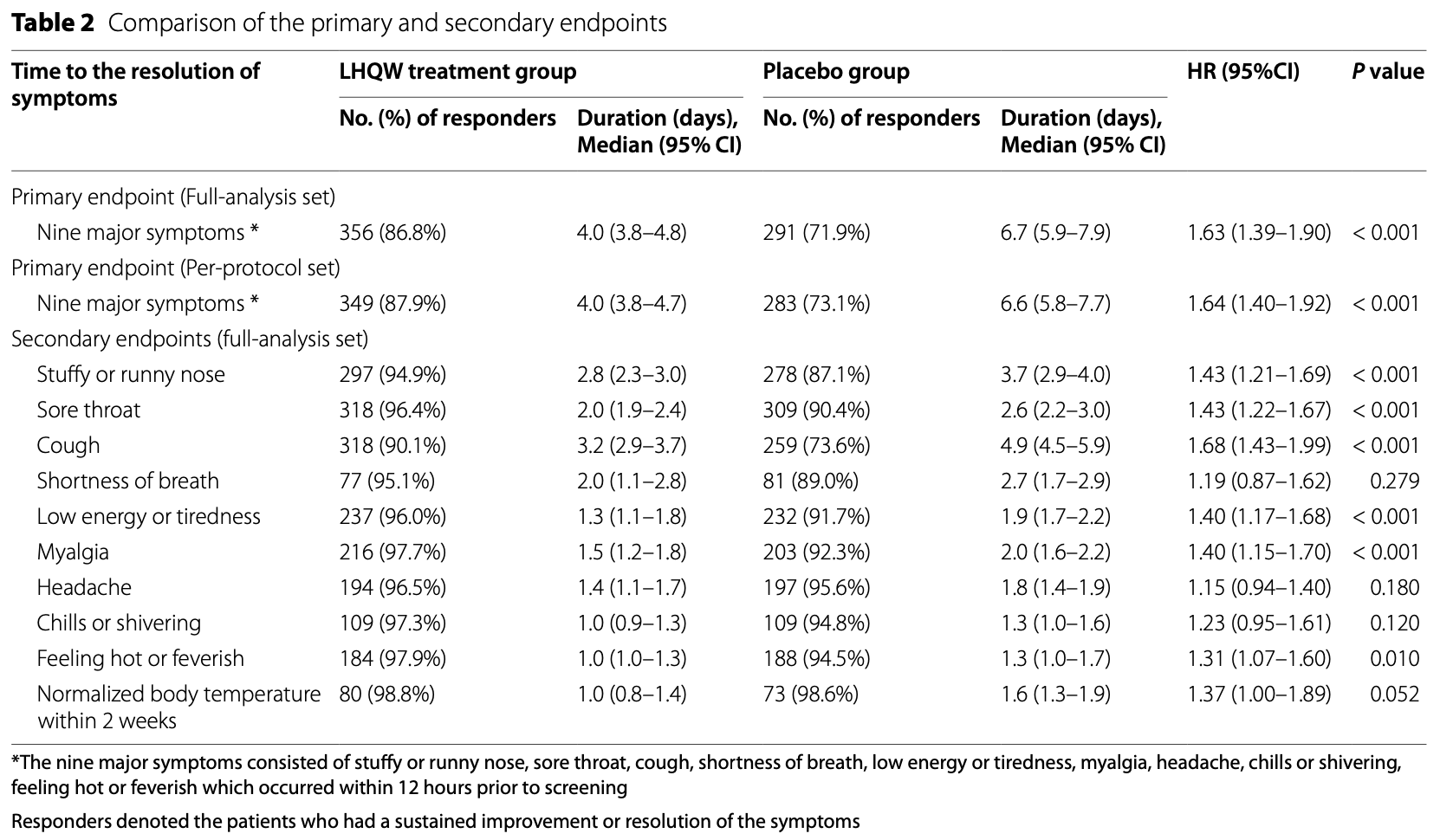
RCT 815 mild-to-moderate COVID-19 patients in China, 410 treated with Lianhuaqingwen (LHQW) for 14 days, showing improved recovery with treatment. 86.8% of the LHQW group achieved symptom resolution by day 14 compared to 71.9% for placebo. No patients progressed to severe disease or died, and safety was comparable between groups. The active ingredient of LHQW include quercetin, kaempferol, luteolin, β-sitosterol, indigo, wogonin, tryptanthrin, [E]-4-phenyl-3-buten-2-one, 1-methyl-2-nonyl-4(1H)-quinolone, stigmasterol, naringenin, and 18β-glycyrrhetinic acid.
Bioavailability. Quercetin has low bioavailability and studies typically use advanced formulations to improve bioavailability which may be required to reach therapeutic concentrations.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
This study is excluded in meta-analysis:
combined treatments may contribute more to the effect seen.
|
risk of no recovery, 38.7% lower, HR 0.61, p < 0.001, treatment 410, control 405, inverted to make HR<1 favor treatment.
|
|
risk of no viral clearance, 10.7% lower, HR 0.89, p = 0.21, treatment 410, control 405, inverted to make HR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Zheng et al., 28 Nov 2023, Double Blind Randomized Controlled Trial, placebo-controlled, China, peer-reviewed, 20 authors, study period February 2022 - December 2022, trial ChiCTR2200056727 (FLOSAN).
Contact: jpzhenggy@163.com, nanshan@vip.163.com.
Effects of Lianhuaqingwen Capsules in adults with mild-to-moderate coronavirus disease 2019: an international, multicenter, double-blind, randomized controlled trial
Virology Journal, doi:10.1186/s12985-023-02144-6
Background In a randomized trial, Lianhuaqingwen (LHQW) capsule was effective for accelerating symptom recovery among patients with coronavirus disease 2019 (COVID-19). However, the lack of blinding and limited sample sizes decreased the level of clinical evidence. Objectives To evaluate the efficacy and safety of LHQW capsule in adults with mild-to-moderate COVID-19.
Methods We conducted a double-blind randomized controlled trial in adults with mild-to-moderate COVID-19 (17 sites from China, Thailand, Philippine and Vietnam). Patients received standard-of-care alone or plus LHQW capsules (4 capsules, thrice daily) for 14 days. The primary endpoint was the median time to sustained clinical improvement or resolution of nine major symptoms.
Results The full-analysis set consisted of 410 patients in LHQW capsules and 405 in placebo group. LHQW significantly shortened the primary endpoint in the full-analysis set (4.0 vs. 6.7 days, hazards ratio: 1.63, 95% confidence interval: 1.39-1.90). LHQW capsules shortened the median time to sustained clinical improvement or resolution of stuffy or runny nose (2.8 vs. 3.7 days), sore throat (2.0 vs. 2.6 days), cough (3.2 vs. 4.9 days), feeling hot or feverish (1.0 vs. 1.3 days), low energy or tiredness (1.3 vs. 1.9 days), and myalgia (1.5 vs. 2.0 days). The duration to sustained clinical improvement or resolution of shortness of breath, headache, and chills or shivering did not differ significantly between the two groups. Safety was comparable between the two groups. No serious adverse events were reported.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s12985-023-02144-6. Additional file 1: Online supplementary tables and figures. Author contributions J. P. Z., Z. F. Y. and N. S. Z. participated in study design; Y. L., L. S. J., P. M., H. Z. L., M. C., L. X. Z., P. A., F. Y. H., T. T. N. L., R. A. P., X. G., H. M. S., J. J. J., R. J. L., Z. D. and Y. Q. Z. recruited patients and performed follow up; W. J. G., J. P. Z. and N. S. Z. drafted the manuscript; J. P. Z. and N. S. Z. were responsible for study conception and provided critical review of the manuscript. All authors read and approved the final manuscript. J. P. Z. and N. S. Z. were the guarantors of the study.
Declarations Ethics approval and consent to participate Ethics approval has been obtained the ethics committee of each participating site, based on Good Clinical Practice. All patients signed written informed consent.
Competing interests None.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Arbel, Sagy, Hoshen, Battat, Lavie et al., Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge, N Engl J Med
Cao, Gao, Bao, Feng, Mei et al., VV116 versus nirmatrelvir-ritonavir for oral treatment of Covid-19, N Engl J Med
Chen, Deng, Fang, Sun, Wu et al., Epidemiological characteristics and transmission dynamics of the outbreak caused by the SARS-CoV-2 Omicron variant in Shanghai, China: a descriptive study, Lancet Reg Health West Pac
Fan, Guo, Yang, Liu, Li et al., Efficacy and safety of Lianhuaqingwen for mild or moderate coronavirus disease 2019: a meta-analysis of randomized controlled trials, Medicine
Gong, Yuan, Yuan, Li, Efficacy and safety of Lianhuaqingwen capsules for the prevention of coronavirus disease 2019: a prospective open-label controlled trial, Evid Based Complement Alternat Med
Gordon, Mouncey, Al-Beidh, Rowan, Nichol et al., Interleukin-6 receptor antagonists in critically Ill patients with Covid-19, N Engl J Med
Guimarães, Quirk, Furtado, Maia, Saraiva et al., Tofacitinib in patients hospitalized with Covid-19 pneumonia, N Engl J Med
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med
Hu, Guan, Bi, Zhang, Li et al., Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial, Phytomedicine
Hui, Ho, Cheung, Ng, Ching et al., SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo, Nature
Jayk, Da Gomes, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med
Kalil, Patterson, Mehta, Tomashek, Wolfe et al., Baricitinib plus remdesivir for hospitalized adults with Covid-19, N Engl J Med
Levin, Ustianowski, Wit, Launay, Avila et al., Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of Covid-19, N Engl J Med
Liu, Gao, Yuan, Yang, Shi et al., Efficacy and safety of herbal medicine (Lianhuaqingwen) for treating COVID-19: a systematic review and meta-analysis, Integr Med Res
Nyberg, Ferguson, Nash, Webster, Flaxman et al., Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study, Lancet
Organization, World Health Organization the 5th interim guidelines for COVID-19
Ruibing, Xin, Clinical observation on 63 cases of suspected cases of new coronavirus pneumonia treated by Chinese medicine Lianhua Qingwen, J Tradit Chin Med
Runfeng, Yunlong, Jicheng, Weiqi, Qinhai et al., Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2), Pharmacol Res
Sun, Zhang, Yang, Chen, Yue et al., Efficacy and safety of Lianhua Qingwen for patients with COVID-19: a systematic review and meta-analysis, Chin J Integr Med
Zhan, Zheng, Efficacy and safety of LianhuaQingwen capsules combined with standard of care in the treatment of adult patients with mild to moderate COVID-19 (FLOSAN): protocol for a randomized, double-blind, international multicenter clinical trial, J Thorac Dis
Zhuang, Dai, Wu, Cai, Fu et al., A meta-analysis for Lianhua Qingwen on the treatment of Coronavirus disease 2019 (COVID-19), Complement Ther Med
DOI record:
{
"DOI": "10.1186/s12985-023-02144-6",
"ISSN": [
"1743-422X"
],
"URL": "http://dx.doi.org/10.1186/s12985-023-02144-6",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>In a randomized trial, Lianhuaqingwen (LHQW) capsule was effective for accelerating symptom recovery among patients with coronavirus disease 2019 (COVID-19). However, the lack of blinding and limited sample sizes decreased the level of clinical evidence.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Objectives</jats:title>\n <jats:p>To evaluate the efficacy and safety of LHQW capsule in adults with mild-to-moderate COVID-19.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We conducted a double-blind randomized controlled trial in adults with mild-to-moderate COVID-19 (17 sites from China, Thailand, Philippine and Vietnam). Patients received standard-of-care alone or plus LHQW capsules (4 capsules, thrice daily) for 14 days. The primary endpoint was the median time to sustained clinical improvement or resolution of nine major symptoms.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>The full-analysis set consisted of 410 patients in LHQW capsules and 405 in placebo group. LHQW significantly shortened the primary endpoint in the full-analysis set (4.0 vs. 6.7 days, hazards ratio: 1.63, 95% confidence interval: 1.39-1.90). LHQW capsules shortened the median time to sustained clinical improvement or resolution of stuffy or runny nose (2.8 vs. 3.7 days), sore throat (2.0 vs. 2.6 days), cough (3.2 vs. 4.9 days), feeling hot or feverish (1.0 vs. 1.3 days), low energy or tiredness (1.3 vs. 1.9 days), and myalgia (1.5 vs. 2.0 days). The duration to sustained clinical improvement or resolution of shortness of breath, headache, and chills or shivering did not differ significantly between the two groups. Safety was comparable between the two groups. No serious adverse events were reported.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Interpretation</jats:title>\n <jats:p>LHQW capsules promote recovery of mild-to-moderate COVID-19 via accelerating symptom resolution and were well tolerated.</jats:p>\n <jats:p><jats:italic>Trial registration</jats:italic><jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/ct2/show/ChiCTR2200056727\">ChiCTR2200056727</jats:ext-link>.</jats:p>\n </jats:sec>",
"alternative-id": [
"2144"
],
"article-number": "277",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "31 May 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "26 July 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "28 November 2023"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Ethics approval has been obtained the ethics committee of each participating site, based on <i>Good Clinical Practice</i>. All patients signed written informed consent."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "None."
}
],
"author": [
{
"affiliation": [],
"family": "Zheng",
"given": "Jin-ping",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ling",
"given": "Yun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jiang",
"given": "Liang-shuang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mootsikapun",
"given": "Piroon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Hong-zhou",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chayakulkeeree",
"given": "Methee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Li-xiu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arttawejkul",
"given": "Pureepat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hu",
"given": "Feng-yu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Truong",
"given": "Thi Ngoc Lan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perez",
"given": "Roxan A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gu",
"given": "Xing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sun",
"given": "Hui-min",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jiang",
"given": "Jian-jie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Ren-jie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ding",
"given": "Zhen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhan",
"given": "Yang-qing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Zi-feng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guan",
"given": "Wei-jie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhong",
"given": "Nan-shan",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "chictr2200056727",
"registry": "10.18810/chictr"
}
],
"container-title": "Virology Journal",
"container-title-short": "Virol J",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
11,
28
]
],
"date-time": "2023-11-28T16:02:58Z",
"timestamp": 1701187378000
},
"deposited": {
"date-parts": [
[
2023,
11,
28
]
],
"date-time": "2023-11-28T16:08:35Z",
"timestamp": 1701187715000
},
"funder": [
{
"award": [
"No: ZYYCXTD-D-202206"
],
"name": "Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine"
},
{
"award": [
"225A2503D"
],
"name": "the Science and Technology Program of Hebei"
}
],
"indexed": {
"date-parts": [
[
2023,
11,
29
]
],
"date-time": "2023-11-29T01:03:53Z",
"timestamp": 1701219833434
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
11,
28
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
28
]
],
"date-time": "2023-11-28T00:00:00Z",
"timestamp": 1701129600000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
28
]
],
"date-time": "2023-11-28T00:00:00Z",
"timestamp": 1701129600000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12985-023-02144-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12985-023-02144-6/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12985-023-02144-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2023,
11,
28
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
28
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "2144_CR1",
"unstructured": "World Health Organization Coronavirus (COVID-19) Dashboard [https://covid19.who.int/]"
},
{
"DOI": "10.1016/S0140-6736(22)00462-7",
"author": "T Nyberg",
"doi-asserted-by": "publisher",
"first-page": "1303",
"issue": "10332",
"journal-title": "Lancet",
"key": "2144_CR2",
"unstructured": "Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, Hinsley W, Bernal JL, Kall M, Bhatt S, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–12.",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/j.lanwpc.2022.100592",
"author": "Z Chen",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Reg Health West Pac",
"key": "2144_CR3",
"unstructured": "Chen Z, Deng X, Fang L, Sun K, Wu Y, Che T, Zou J, Cai J, Liu H, Wang Y, et al. Epidemiological characteristics and transmission dynamics of the outbreak caused by the SARS-CoV-2 Omicron variant in Shanghai, China: a descriptive study. Lancet Reg Health West Pac. 2022;29: 100592.",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2204919",
"author": "R Arbel",
"doi-asserted-by": "publisher",
"first-page": "790",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "2144_CR4",
"unstructured": "Arbel R, Wolff Sagy Y, Hoshen M, Battat E, Lavie G, Sergienko R, Friger M, Waxman JG, Dagan N, Balicer R, et al. Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge. N Engl J Med. 2022;387(9):790–8.",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"author": "BA Jayk",
"doi-asserted-by": "publisher",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "2144_CR5",
"unstructured": "Jayk BA, da Gomes SMM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos RV, Martín-Quirós A, Caraco Y, Williams-Diaz A, Brown ML, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–20.",
"volume": "386",
"year": "2022"
},
{
"author": "Z Cao",
"first-page": "21",
"journal-title": "N Engl J Med",
"key": "2144_CR6",
"unstructured": "Cao Z, Gao W, Bao H, Feng H, Mei S, Chen P, Gao Y, Cui Z, Zhang Q, Meng X, et al. VV116 versus nirmatrelvir-ritonavir for oral treatment of Covid-19. N Engl J Med. 2022;5:21.",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116620",
"author": "MJ Levin",
"doi-asserted-by": "publisher",
"first-page": "2188",
"issue": "23",
"journal-title": "N Engl J Med",
"key": "2144_CR7",
"unstructured": "Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A, Yuan Y, Seegobin S, Ellery A, Levinson DJ, et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of Covid-19. N Engl J Med. 2022;386(23):2188–200.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2107934",
"author": "A Gupta",
"doi-asserted-by": "publisher",
"first-page": "1941",
"issue": "21",
"journal-title": "N Engl J Med",
"key": "2144_CR8",
"unstructured": "Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci DR, Sarkis E, Solis J, Zheng H, Scott N, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–50.",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2100433",
"author": "AC Gordon",
"doi-asserted-by": "publisher",
"first-page": "1491",
"issue": "16",
"journal-title": "N Engl J Med",
"key": "2144_CR9",
"unstructured": "Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, van Bentum-Puijk W, Berry LR, et al. Interleukin-6 receptor antagonists in critically Ill patients with Covid-19. N Engl J Med. 2021;384(16):1491–502.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2101643",
"author": "PO Guimarães",
"doi-asserted-by": "publisher",
"first-page": "406",
"issue": "5",
"journal-title": "N Engl J Med",
"key": "2144_CR10",
"unstructured": "Guimarães PO, Quirk D, Furtado RH, Maia LN, Saraiva JF, Antunes MO, Kalil Filho R, Junior VM, Soeiro AM, Tognon AP, et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385(5):406–15.",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031994",
"author": "AC Kalil",
"doi-asserted-by": "publisher",
"first-page": "795",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "2144_CR11",
"unstructured": "Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807.",
"volume": "384",
"year": "2021"
},
{
"key": "2144_CR12",
"unstructured": "Ministry of Health C: the Protocol of the Diagnosis and Treatment of Coronavirus Disease 2019. In: 2023"
},
{
"DOI": "10.1016/j.phrs.2020.104761",
"author": "L Runfeng",
"doi-asserted-by": "publisher",
"first-page": "104761",
"journal-title": "Pharmacol Res",
"key": "2144_CR13",
"unstructured": "Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, Yongxia S, Chufang L, Jin Z, Zhenhua J, Haiming J, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res. 2020;156:104761.",
"volume": "156",
"year": "2020"
},
{
"author": "LWW Ruibing",
"first-page": "655",
"issue": "08",
"journal-title": "J Tradit Chin Med",
"key": "2144_CR14",
"unstructured": "Ruibing LWW, Xin L. Clinical observation on 63 cases of suspected cases of new coronavirus pneumonia treated by Chinese medicine Lianhua Qingwen. J Tradit Chin Med. 2020;61(08):655–9.",
"volume": "61",
"year": "2020"
},
{
"DOI": "10.1016/j.phymed.2020.153242",
"author": "K Hu",
"doi-asserted-by": "publisher",
"first-page": "153242",
"journal-title": "Phytomedicine",
"key": "2144_CR15",
"unstructured": "Hu K, Guan WJ, Bi Y, Zhang W, Li L, Zhang B, Liu Q, Song Y, Li X, Duan Z, et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine. 2021;85:153242.",
"volume": "85",
"year": "2021"
},
{
"DOI": "10.21037/jtd-23-281",
"author": "YQCR Zhan",
"doi-asserted-by": "publisher",
"first-page": "2859",
"issue": "5",
"journal-title": "J Thorac Dis",
"key": "2144_CR16",
"unstructured": "Zhan YQCR, Zheng QS, et al. Efficacy and safety of LianhuaQingwen capsules combined with standard of care in the treatment of adult patients with mild to moderate COVID-19 (FLOSAN): protocol for a randomized, double-blind, international multicenter clinical trial. J Thorac Dis. 2023;15(5):2859–72.",
"volume": "15",
"year": "2023"
},
{
"key": "2144_CR17",
"unstructured": "Organization WH: World Health Organization the 5th interim guidelines for COVID-19. In.: World Health Organization; 2020."
},
{
"DOI": "10.1038/s41586-022-04479-6",
"author": "KPY Hui",
"doi-asserted-by": "publisher",
"first-page": "715",
"issue": "7902",
"journal-title": "Nature",
"key": "2144_CR18",
"unstructured": "Hui KPY, Ho JCW, Cheung MC, Ng KC, Ching RHH, Lai KL, Kam TT, Gu H, Sit KY, Hsin MKY, et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603(7902):715–20.",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1007/s11655-022-3578-8",
"author": "XH Sun",
"doi-asserted-by": "publisher",
"first-page": "650",
"issue": "7",
"journal-title": "Chin J Integr Med",
"key": "2144_CR19",
"unstructured": "Sun XH, Zhang S, Yang Z, Chen ZL, Yue SJ, Zhang S, Tang YP. Efficacy and safety of Lianhua Qingwen for patients with COVID-19: a systematic review and meta-analysis. Chin J Integr Med. 2022;28(7):650–60.",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/j.ctim.2021.102754",
"author": "J Zhuang",
"doi-asserted-by": "publisher",
"first-page": "102754",
"journal-title": "Complement Ther Med",
"key": "2144_CR20",
"unstructured": "Zhuang J, Dai X, Wu Q, Cai H, Fu X, Zhang W, Chen B. A meta-analysis for Lianhua Qingwen on the treatment of Coronavirus disease 2019 (COVID-19). Complement Ther Med. 2021;60:102754.",
"volume": "60",
"year": "2021"
},
{
"DOI": "10.1097/MD.0000000000026059",
"author": "Z Fan",
"doi-asserted-by": "publisher",
"first-page": "e26059",
"issue": "21",
"journal-title": "Medicine (Baltimore)",
"key": "2144_CR21",
"unstructured": "Fan Z, Guo G, Che X, Yang Y, Liu Y, Li L, Chang X, Han L, Cai X, Tang H. Efficacy and safety of Lianhuaqingwen for mild or moderate coronavirus disease 2019: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2021;100(21):e26059.",
"volume": "100",
"year": "2021"
},
{
"DOI": "10.1016/j.imr.2020.100644",
"author": "M Liu",
"doi-asserted-by": "publisher",
"first-page": "100644",
"issue": "1",
"journal-title": "Integr Med Res",
"key": "2144_CR22",
"unstructured": "Liu M, Gao Y, Yuan Y, Yang K, Shi S, Tian J, Zhang J. Efficacy and safety of herbal medicine (Lianhuaqingwen) for treating COVID-19: a systematic review and meta-analysis. Integr Med Res. 2021;10(1):100644.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1155/2021/7962630",
"author": "X Gong",
"doi-asserted-by": "publisher",
"first-page": "7962630",
"journal-title": "Evid Based Complement Alternat Med",
"key": "2144_CR23",
"unstructured": "Gong X, Yuan B, Yuan Y, Li F. Efficacy and safety of Lianhuaqingwen capsules for the prevention of coronavirus disease 2019: a prospective open-label controlled trial. Evid Based Complement Alternat Med. 2021;2021:7962630.",
"volume": "2021",
"year": "2021"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://virologyj.biomedcentral.com/articles/10.1186/s12985-023-02144-6"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Effects of Lianhuaqingwen Capsules in adults with mild-to-moderate coronavirus disease 2019: an international, multicenter, double-blind, randomized controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "20"
}
