A first-in-human clinical study of an intranasal spray of a cocktail containing two synergetic antibodies neutralizes Omicron BA. 4/5
et al., medRxiv, doi:10.1101/2023.03.17.23287398, ChiCTR2200066525, Mar 2023
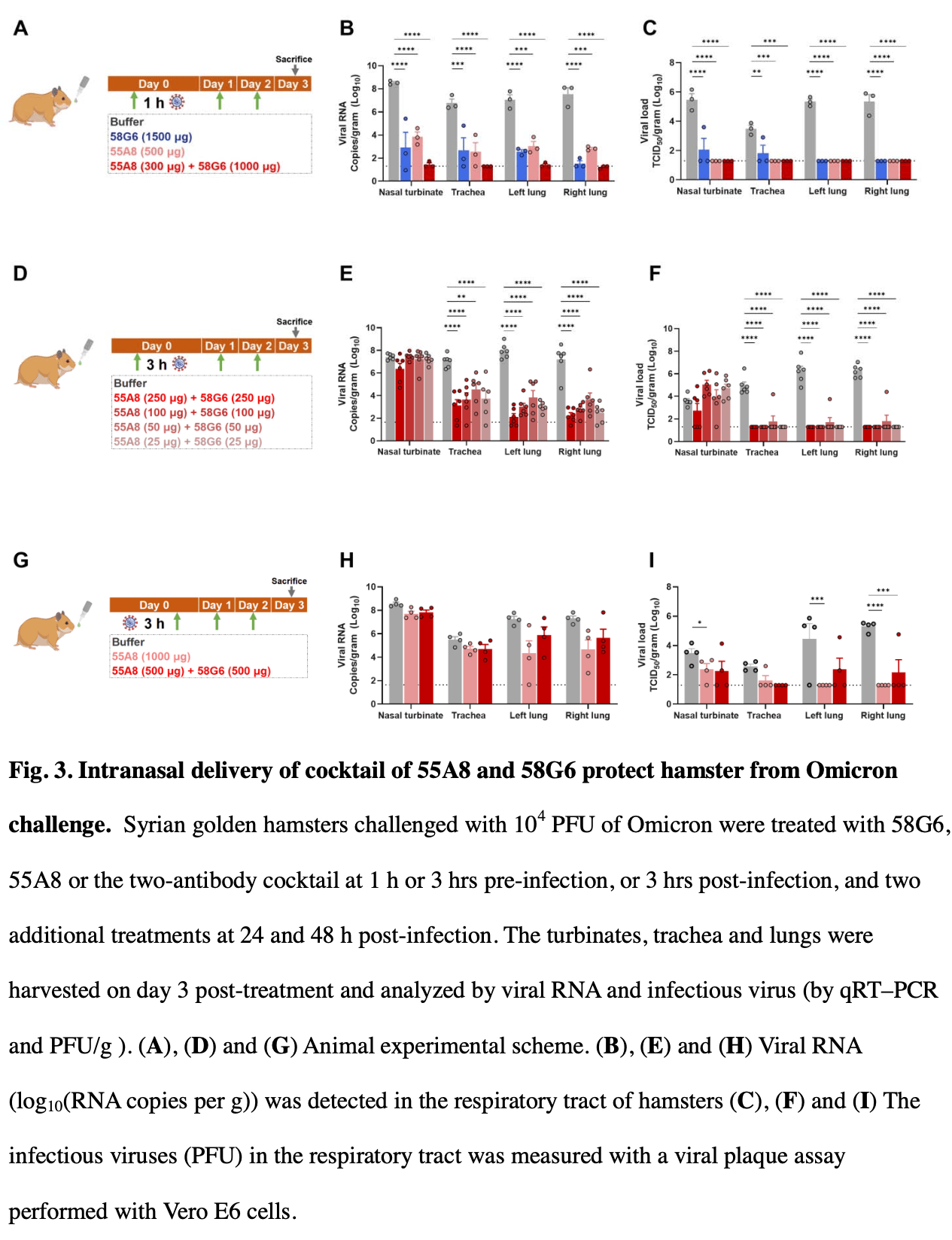
In vitro and animal study showing that a cocktail of two synergetic antibodies (55A8 and 58G6) neutralizes SARS-CoV-2 omicron variants and provides protection against infection when delivered intranasally. Authors found that the antibody cocktail broadly neutralized SARS-CoV-2 variants, including omicron BA.1, BA.1.1, BA.2, BA.2.12.1, and BA.5, in pseudovirus and authentic virus neutralization assays. Cryo-EM structural analysis revealed a unique synergetic neutralizing mechanism where 55A8 and 58G6 bind to exclusive epitopes on the receptor-binding domain (RBD) and structurally compensate each other in blocking the spike-ACE2 interaction. In hamsters challenged with omicron BA.1, intranasal administration of the antibody cocktail at low doses (25 μg each) significantly reduced viral loads in the respiratory tract. A clinical trial in 108 healthy volunteers demonstrated that the antibody cocktail nasal spray was safe and well-tolerated, with nasal concentrations remaining above the IC90 for BA.4/5 neutralization for at least 24 hours after dosing. Ex vivo neutralization assays confirmed potent activity against omicron BA.4/5 in nasal samples from study subjects.
Zhang et al., 20 Mar 2023, preprint, 36 authors, trial ChiCTR2200066525.
Contact: yanght@shanghaitech.edu.cn, aishunjin@cqmu.edu.cn, xiaoyun.ji@nju.edu.cn, gongr@wh.iov.cn, qiux@ustc.edu.cn, ahuang@cqmu.edu.cn.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
A first-in-human clinical study of an intranasal spray of a cocktail containing two synergetic antibodies neutralizes Omicron BA.4/5
doi:10.1101/2023.03.17.23287398
All rights reserved. No reuse allowed without permission. (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
Author contributions:
References
Andrews, Stowe, Kirsebom, Toffa, Sachdeva et al., Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England, Nature medicine
Andrews, Tessier, Stowe, Gower, Kirsebom et al., None
Bequignon, Dhommée, Angely, Thomas, Bottier et al., FcRn-Dependent Transcytosis of Monoclonal Antibody in Human Nasal Epithelial Cells In Vitro: A Prerequisite for a New Delivery Route for Therapy, International journal of molecular sciences
Burki, Omicron variant and booster COVID-19 vaccines, The Lancet. Respiratory medicine
Burki, The future of Paxlovid for COVID-19, The Lancet. Respiratory medicine
Cao, Yisimayi, Jian, Song, Xiao et al., None
Carvalho, Silent spread, Nature medicine
Costa Clemens, Weckx, Clemens, Almeida Mendes, Souza et al., Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study, Lancet
Dolgin, The race for antiviral drugs to beat COVID -and the next pandemic, Nature
Emsley, Lohkamp, Scott, Cowtan, Features and development of Coot, Acta Crystallographica Section D
Fan, Sun, Zhang, Zhang, Jiao et al., Nasal delivery of thermostable and broadly neutralizing antibodies protects mice against SARS-CoV-2 infection, Signal transduction and targeted therapy
Feng, Wang, Shan, Yang, Feng et al., An adenovirusvectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques, Nature communications
Gao, Huang, Li, Hu, Shen et al., A Highly Conserved Peptide Vaccine Candidate Activates Both Humoral and Cellular Immunity Against SARS-CoV-2 Variant Strains, Frontiers in immunology
Garrett, Tapley, Andriesen, Seocharan, Fisher et al., High Asymptomatic Carriage with the Omicron Variant in South Africa
Garrett, Tapley, Andriesen, Seocharan, Fisher et al., High Rate of Asymptomatic Carriage Associated with Variant Strain Omicron
Graham, Mascola, Chang, Yin, Sobieszczyk et al., Antibody resistance of SARS-CoV-2 variants B.1.351 and, Nature
Green, Matochko, Thomson, Vögeli, Krüger et al., Multivalent designed proteins neutralize SARS-CoV-2 variants of concern and confer protection against infection in mice, Science translational medicine
Groves, Dabrera, Myers, Campbell, Amirthalingam et al., Duration of Protection against Mild and Severe Disease by Covid-19 Vaccines, The New England journal of medicine
Guo, Gao, Li, Li, Lu et al., Structures of Omicron spike complexes and implications for neutralizing antibody development, Cell reports
Han, Wang, Li, Hu, Li et al., A Rapid and Efficient Screening System for Neutralizing Antibodies and Its Application for SARS-CoV-2, Frontiers in immunology
Hui, Ho, Cheung, Ng, Ching et al., SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo, Nature
Hunt, Case, Park, Cao, Wu et al., None
Ibrahim, Verma, Garcia-Contreras, Inhalation drug delivery devices: technology update. Medical devices
Kelley, Mezulis, Yates, Wass, Sternberg, The Phyre2 web portal for protein modeling, prediction and analysis, Nature Protocols
Kimura, Kosugi, Wu, Zahradnik, Yamasoba et al., The SARS-CoV-2 Lambda variant exhibits enhanced infectivity and immune resistance, Cell reports
Ku, Xie, Davidson, Ye, Su et al., Molecular determinants and mechanism for antibody cocktail preventing SARS-CoV-2 escape, Nature communications
Ku, Xie, Hinton, Liu, Ye et al., Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants, Nature
Kuhlmann, Mayer, Claassen, Maponga, Burgers et al., Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose, Lancet
Levin, Ustianowski, De Wit, Launay, Avila et al., Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19, The New England journal of medicine
Li, Gu, Shao, Huang, Jin et al., 4 and BA.5 escape antibodies elicited by Omicron infection, Nature
Li, Han, Gu, Guo, Zhang et al., Potent SARS-CoV-2 neutralizing antibodies with protective efficacy against newly emerged mutational variants, Nature communications
Li, Sempowski, Saunders, Acharya, Haynes, SARS-CoV-2 Neutralizing Antibodies for COVID-19 Prevention and Treatment, Annual review of medicine
Liebschner, Afonine, Baker, Bunkoczi, Chen et al., Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix, Acta Crystallographica Section D
Loo, Mctamney, Arends, Abram, Aksyuk et al., The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans, Science translational medicine
Mahase, Covid-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports, BMJ (Clinical research ed
Mastronarde, Automated electron microscope tomography using robust prediction of specimen movements, J Struct Biol
Mcknight, Altmann, Boyton, Abbass, Abiodun et al., Omicron) depends on previous SARS-CoV-2 exposure
Meng, Abdullahi, Ferreira, Goonawardane, Saito et al., Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity, Nature
Mlcochova, Kemp, Dhar, Papa, Meng et al., SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion, Nature
Ojaimi, Blin, Lamamy, Gracia, Pitiot et al., Therapeutic antibodies -natural and pathological barriers and strategies to overcome them, Pharmacology & therapeutics
Ou, Zhang, Wang, Zhang, Wei et al., ACE2-Targeting antibody suppresses SARS-CoV-2 Omicron and Delta variants, Signal transduction and targeted therapy
Parray, Shukla, Perween, Khatri, Shrivastava et al., Inhalation monoclonal antibody therapy: a new way to treat and manage respiratory infections, Applied microbiology and biotechnology
Pettersen, Goddard, Huang, Couch, Greenblatt et al., UCSF Chimera-A visualization system for exploratory research and analysis, Journal of Computational Chemistry
Phizackerley, Three more points about Paxlovid for covid-19, BMJ (Clinical research ed
Punjani, Rubinstein, Fleet, Brubaker, cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination, Nature Methods
Reynolds, Pade, Gibbons, Otter, Lin et al., None
Rockett, Basile, Maddocks, Fong, Agius et al., Resistance Mutations in SARS-CoV-2 Delta Variant after Sotrovimab Use, The New England journal of medicine
Rubin, Baden, Morrissey, Audio Interview: A Potential New Agent to Treat Covid-19, The New England journal of medicine
Rubin, Monoclonal Antibodies for COVID-19 Preexposure Prophylaxis Can't Come Fast Enough for Some People, Jama
Rubin, Questions Remain About Who Will Get Monoclonal Antibodies for COVID-19 Preexposure Prophylaxis, Jama
Shapira, Monreal, Dion, Buchholz, Imbiakha et al., A TMPRSS2 inhibitor acts as a pan-SARS-CoV-2 prophylactic and therapeutic, Nature
Sockolosky, Tiffany, Szoka, Engineering neonatal Fc receptor-mediated recycling and transcytosis in recombinant proteins by short terminal peptide extensions, Proceedings of the National Academy of Sciences of the United States of America
Sonnleitner, Prelog, Sonnleitner, Hinterbichler, Halbfurter et al., Cumulative SARS-CoV-2 mutations and corresponding changes in immunity in an immunocompromised patient indicate viral evolution within the host, Nature communications
Stuver, Shah, Korde, Roeker, Mato et al., Activity of AZD7442 (tixagevimab-cilgavimab) against Omicron SARS-CoV-2 in patients with hematologic malignancies, Cancer cell
Wang, Casner, Nair, Wang, Yu et al., Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization, Cell host & microbe
Wang, Nair, Liu, Iketani, Luo et al., None
Wu, Cheng, Fu, Huang, Zhu et al., A potent bispecific nanobody protects hACE2 mice against SARS-CoV-2 infection via intranasal administration, Cell reports
Zhang, Li, Zhang, Zhang, Zhang, A mouse model for SARS-CoV-2 infection by exogenous delivery of hACE2 using alphavirus replicon particles, Cell Research
Zhang, Zhang, Li, Chen, Luo et al., A potent neutralizing antibody provides protection against SARS-CoV-2 Omicron and Delta variants via nasal delivery, Signal transduction and targeted therapy
Zhao, Lu, Peng, Chen, Meng et al., SARS-CoV-2 Omicron variant shows less efficient replication and fusion activity when compared with Delta variant in TMPRSS2-expressed cells, Emerging microbes & infections
Zhao, Zhang, Yang, Zhang, Chen et al., Identification of potent human neutralizing antibodies against SARS-CoV-2 implications for development of therapeutics and prophylactics, Nature communications
Zheng, Palovcak, Armache, Verba, Cheng et al., MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy, Nature Methods
Zhou, Yang, Wang, Hu, Zhang et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature
Zhu, Lee, Woo, Xu, Nguyenla et al., An intranasal ASO therapeutic targeting SARS-CoV-2, Nature communications
DOI record:
{
"DOI": "10.1101/2023.03.17.23287398",
"URL": "http://dx.doi.org/10.1101/2023.03.17.23287398",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Neutralizing monoclonal antibodies (NAbs) with prophylactic and therapeutic efficacy have demonstrated fundamental importance in the control of SARS-CoV-2 transmission. However, their wide application has been largely limited by high cost and inconvenience in administration. Here, we developed an intranasal spray containing two synergetic human NAbs that could broadly neutralize the emerging Omicron variants in vitro. A unique synergetic neutralizing mechanism was identified that the two NAbs bound to exclusive epitopes on the RBD and structurally compensate each other in blocking the Spike-ACE2 interaction. Importantly, when given at low dosages for three consecutive days through the intranasal mucosal route, this cocktail showed significant improvement in the emergency preventive and therapeutic effects in hamsters challenged with authentic Omicron BA.1. Further, we performed an investigator-initiated trail in healthy volunteers (ChiCTR2200066525) to study the safety and pharmacokinetics of the antibody cocktail administrated as nasal spray. The nasal spray is generally safe and well tolerated without treatment related severe abnormal effects. The antibody cocktail nasal spray demonstrated nasal concentrations higher than the IC<jats:sub>90</jats:sub>of neutralization activity against Omicron BA.4/5 even at 24 hours post dosing. Furthermore, nasal samples from the study subjects demonstrated potent neutralization activity against Omicron BA.4/5 in an ex vivo pseudovirus neutralization assay. Together, we provide a novel approach for NAb regimens, a potentially highly effective product with broad applicable perspective in depressing the infection risk of new epidemic variant and ameliorating the heavy medical burden of hospital.</jats:p><jats:sec><jats:title>One Sentence Summary</jats:title><jats:p>An intranasal spray of two synergetic antibodies cocktail neutralizing Omicron BA.4/5 and an initial clinical evaluation in healthy volunteers.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2023,
3,
20
]
]
},
"author": [
{
"affiliation": [],
"family": "Zhang",
"given": "Xinghai",
"sequence": "first"
},
{
"affiliation": [],
"family": "Luo",
"given": "Feiyang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Huajun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guo",
"given": "Hangtian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhou",
"given": "Junhui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Tingting",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Shaohong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Song",
"given": "Shuyi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shen",
"given": "Meiying",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wu",
"given": "Yan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gao",
"given": "Yan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Han",
"given": "Xiaojian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Yingming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hu",
"given": "Chao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Xiaodong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guo",
"given": "Huilin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Dazhi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Yuchi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Wei",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0137-1247",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wang",
"given": "Kai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tang",
"given": "Ni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jin",
"given": "Tengchuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ding",
"given": "Menglu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luo",
"given": "Shuhui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "Cuicui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Tingting",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Bingxia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tian",
"given": "Yang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Chengyong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "Guofeng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Haitao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jin",
"given": "Aishun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ji",
"given": "Xiaoyun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gong",
"given": "Rui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chiu",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Ailong",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
3,
20
]
],
"date-time": "2023-03-20T17:00:13Z",
"timestamp": 1679331613000
},
"deposited": {
"date-parts": [
[
2023,
3,
22
]
],
"date-time": "2023-03-22T20:10:48Z",
"timestamp": 1679515848000
},
"group-title": "Respiratory Medicine",
"indexed": {
"date-parts": [
[
2023,
3,
23
]
],
"date-time": "2023-03-23T04:51:44Z",
"timestamp": 1679547104300
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
3,
20
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2023.03.17.23287398",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2023,
3,
20
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2023,
3,
20
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1038/s41586-021-03944-y",
"doi-asserted-by": "publisher",
"key": "2023032213100791000_2023.03.17.23287398v1.1"
},
{
"DOI": "10.1056/NEJMc2031364",
"doi-asserted-by": "publisher",
"key": "2023032213100791000_2023.03.17.23287398v1.2"
},
{
"DOI": "10.1016/j.chom.2021.04.007",
"article-title": "Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization",
"doi-asserted-by": "crossref",
"first-page": "747",
"journal-title": "Cell host & microbe",
"key": "2023032213100791000_2023.03.17.23287398v1.3",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.1038/s41586-022-04474-x",
"article-title": "Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity",
"doi-asserted-by": "crossref",
"first-page": "706",
"journal-title": "Nature",
"key": "2023032213100791000_2023.03.17.23287398v1.4",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1080/22221751.2021.2023329",
"article-title": "SARS-CoV-2 Omicron variant shows less efficient replication and fusion activity when compared with Delta variant in TMPRSS2-expressed cells",
"doi-asserted-by": "crossref",
"first-page": "277",
"journal-title": "Emerging microbes & infections",
"key": "2023032213100791000_2023.03.17.23287398v1.5",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04479-6",
"doi-asserted-by": "publisher",
"key": "2023032213100791000_2023.03.17.23287398v1.6"
},
{
"DOI": "10.1038/s41591-020-01165-w",
"article-title": "Silent spread",
"doi-asserted-by": "crossref",
"first-page": "1807",
"journal-title": "Nature medicine",
"key": "2023032213100791000_2023.03.17.23287398v1.7",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1101/2021.12.20.21268130",
"doi-asserted-by": "crossref",
"key": "2023032213100791000_2023.03.17.23287398v1.8",
"unstructured": "N. Garrett , A. Tapley , J. Andriesen , I. Seocharan , L. H. Fisher , L. Bunts , N. Espy , C. L. Wallis , A. K. Randhawa , N. Ketter , M. Yacovone , A. Goga , L. G. Bekker , G. E. Gray , L. Corey , High Rate of Asymptomatic Carriage Associated with Variant Strain Omicron. medRxiv : the preprint server for health sciences, (2022)."
},
{
"key": "2023032213100791000_2023.03.17.23287398v1.9",
"unstructured": "N. Garrett , A. Tapley , J. Andriesen , I. Seocharan , L. H. Fisher , L. Bunts , N. Espy , C. L. Wallis , A. K. Randhawa , M. D. Miner , N. Ketter , M. Yacovone , A. Goga , Y. Huang , J. Hural , P. Kotze , L. G. Bekker , G. E. Gray , L. Corey , High Asymptomatic Carriage with the Omicron Variant in South Africa. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, (2022)."
},
{
"DOI": "10.1056/NEJMOA2115481",
"doi-asserted-by": "publisher",
"key": "2023032213100791000_2023.03.17.23287398v1.10"
},
{
"DOI": "10.1038/s41591-022-01699-1",
"article-title": "Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England",
"doi-asserted-by": "crossref",
"first-page": "831",
"journal-title": "Nature medicine",
"key": "2023032213100791000_2023.03.17.23287398v1.11",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(21)00559-2",
"article-title": "Omicron variant and booster COVID-19 vaccines",
"doi-asserted-by": "crossref",
"first-page": "e17",
"journal-title": "The Lancet. Respiratory medicine",
"key": "2023032213100791000_2023.03.17.23287398v1.12",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00090-3",
"article-title": "Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose",
"doi-asserted-by": "crossref",
"first-page": "625",
"journal-title": "Lancet (London, England)",
"key": "2023032213100791000_2023.03.17.23287398v1.13",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00094-0",
"article-title": "Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study",
"doi-asserted-by": "crossref",
"first-page": "521",
"journal-title": "Lancet (London, England)",
"key": "2023032213100791000_2023.03.17.23287398v1.14",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1126/science.abq1841",
"doi-asserted-by": "crossref",
"key": "2023032213100791000_2023.03.17.23287398v1.15",
"unstructured": "C. J. Reynolds , C. Pade , J. M. Gibbons , A. D. Otter , K. M. Lin , D. Muñoz Sandoval , F. P. Pieper , D. K. Butler , S. Liu , G. Joy , N. Forooghi , T. A. Treibel , C. Manisty , J. C. Moon , A. Semper , T. Brooks , Á. McKnight , D. M. Altmann , R. J. Boyton , H. Abbass , A. Abiodun , M. Alfarih , Z. Alldis , D. M. Altmann , O. E. Amin , M. Andiapen , J. Artico , J. B. Augusto , G. L. Baca , S. N. L. Bailey , A. N. Bhuva , A. Boulter , R. Bowles , R. J. Boyton , O. V. Bracken , B. O’Brien , T. Brooks , N. Bullock , D. K. Butler , G. Captur , O. Carr , N. Champion , C. Chan , A. Chandran , T. Coleman , J. Couto de Sousa , X. Couto-Parada , E. Cross , T. Cutino-Moguel , S. D’Arcangelo , R. H. Davies , B. Douglas , C. Di Genova , K. Dieobi-Anene , M. O. Diniz , A. Ellis , K. Feehan , M. Finlay , M. Fontana , N. Forooghi , S. Francis , J. M. Gibbons , D. Gillespie , D. Gilroy , M. Hamblin , G. Harker , G. Hemingway , J. Hewson , W. Heywood , L. M. Hickling , B. Hicks , A. D. Hingorani , L. Howes , I. Itua , V. Jardim , W. J. Lee , M. Jensen , J. Jones , M. Jones , G. Joy , V. Kapil , C. Kelly , H. Kurdi , J. Lambourne , K. M. Lin , S. Liu , A. Lloyd , S. Louth , M. K. Maini , V. Mandadapu , C. Manisty , Á. McKnight , K. Menacho , C. Mfuko , K. Mills , S. Millward , O. Mitchelmore , C. Moon , J. Moon , D. Muñoz Sandoval , S. M. Murray , M. Noursadeghi , A. Otter , C. Pade , S. Palma , R. Parker , K. Patel , M. Pawarova , S. E. Petersen , B. Piniera , F. P. Pieper , L. Rannigan , A. Rapala , C. J. Reynolds , A. Richards , M. Robathan , J. Rosenheim , C. Rowe , M. Royds , J. Sackville West , G. Sambile , N. M. Schmidt , H. Selman , A. Semper , A. Seraphim , M. Simion , A. Smit , M. Sugimoto , L. Swadling , S. Taylor , N. Temperton , S. Thomas , G. D. Thornton , T. A. Treibel , A. Tucker , A. Varghese , J. Veerapen , M. Vijayakumar , T. Warner , S. Welch , H. White , T. Wodehouse , L. Wynne , D. Zahedi , B. Chain , J. C. Moon , Immune boosting by B.1.1.529 (Omicron) depends on previous SARS-CoV-2 exposure. Science (New York, N.Y.), eabq1841 (2022)."
},
{
"DOI": "10.1101/2022.04.30.489997",
"doi-asserted-by": "crossref",
"key": "2023032213100791000_2023.03.17.23287398v1.16",
"unstructured": "Y. Cao , A. Yisimayi , F. Jian , W. Song , T. Xiao , L. Wang , S. Du , J. Wang , Q. Li , X. Chen , Y. Yu , P. Wang , Z. Zhang , P. Liu , R. An , X. Hao , Y. Wang , J. Wang , R. Feng , H. Sun , L. Zhao , W. Zhang , D. Zhao , J. Zheng , L. Yu , C. Li , N. Zhang , R. Wang , X. Niu , S. Yang , X. Song , Y. Chai , Y. Hu , Y. Shi , L. Zheng , Z. Li , Q. Gu , F. Shao , W. Huang , R. Jin , Z. Shen , Y. Wang , X. Wang , J. Xiao , X. S. Xie , BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature, (2022)."
},
{
"DOI": "10.1136/bmj.n2713",
"doi-asserted-by": "publisher",
"key": "2023032213100791000_2023.03.17.23287398v1.17"
},
{
"DOI": "10.1016/S2213-2600(22)00192-8",
"doi-asserted-by": "crossref",
"key": "2023032213100791000_2023.03.17.23287398v1.18",
"unstructured": "T. Burki , The future of Paxlovid for COVID-19. The Lancet. Respiratory medicine, (2022)."
},
{
"DOI": "10.1136/bmj.o1397",
"doi-asserted-by": "publisher",
"key": "2023032213100791000_2023.03.17.23287398v1.19"
},
{
"DOI": "10.1016/S1473-3099(21)00633-2",
"article-title": "Unmet need for COVID-19 therapies in community settings",
"doi-asserted-by": "crossref",
"first-page": "1471",
"journal-title": "The Lancet. Infectious diseases",
"key": "2023032213100791000_2023.03.17.23287398v1.20",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1038/d41586-021-00958-4",
"doi-asserted-by": "publisher",
"key": "2023032213100791000_2023.03.17.23287398v1.21"
},
{
"DOI": "10.1056/NEJMe2119792",
"article-title": "Audio Interview: A Potential New Agent to Treat Covid-19",
"doi-asserted-by": "crossref",
"first-page": "e101",
"journal-title": "The New England journal of medicine",
"key": "2023032213100791000_2023.03.17.23287398v1.22",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMOA2116620",
"doi-asserted-by": "publisher",
"key": "2023032213100791000_2023.03.17.23287398v1.23"
},
{
"DOI": "10.1126/scitranslmed.abl8124",
"article-title": "The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans",
"doi-asserted-by": "crossref",
"first-page": "eabl8124",
"journal-title": "Science translational medicine",
"key": "2023032213100791000_2023.03.17.23287398v1.24",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1016/j.ccell.2022.05.007",
"article-title": "Activity of AZD7442 (tixagevimab-cilgavimab) against Omicron SARS-CoV-2 in patients with hematologic malignancies",
"doi-asserted-by": "crossref",
"first-page": "590",
"journal-title": "Cancer cell",
"key": "2023032213100791000_2023.03.17.23287398v1.25",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.23557",
"doi-asserted-by": "publisher",
"key": "2023032213100791000_2023.03.17.23287398v1.26"
},
{
"DOI": "10.1001/jama.2021.19534",
"article-title": "Monoclonal Antibodies for COVID-19 Preexposure Prophylaxis Can’t Come Fast Enough for Some People",
"doi-asserted-by": "crossref",
"first-page": "1895",
"journal-title": "Jama",
"key": "2023032213100791000_2023.03.17.23287398v1.27",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1126/scitranslmed.abn1252",
"article-title": "Multivalent designed proteins neutralize SARS-CoV-2 variants of concern and confer protection against infection in mice",
"doi-asserted-by": "crossref",
"first-page": "eabn1252",
"journal-title": "Science translational medicine",
"key": "2023032213100791000_2023.03.17.23287398v1.28",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1038/s41392-022-00911-5",
"article-title": "Nasal delivery of thermostable and broadly neutralizing antibodies protects mice against SARS-CoV-2 infection",
"doi-asserted-by": "crossref",
"first-page": "55",
"journal-title": "Signal transduction and targeted therapy",
"key": "2023032213100791000_2023.03.17.23287398v1.29",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1038/s41392-022-00913-3",
"article-title": "ACE2-Targeting antibody suppresses SARS-CoV-2 Omicron and Delta variants",
"doi-asserted-by": "crossref",
"first-page": "43",
"journal-title": "Signal transduction and targeted therapy",
"key": "2023032213100791000_2023.03.17.23287398v1.30",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1038/s41392-022-01135-3",
"article-title": "A potent neutralizing antibody provides protection against SARS-CoV-2 Omicron and Delta variants via nasal delivery",
"doi-asserted-by": "crossref",
"first-page": "301",
"journal-title": "Signal transduction and targeted therapy",
"key": "2023032213100791000_2023.03.17.23287398v1.31",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-03673-2",
"article-title": "Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants",
"doi-asserted-by": "crossref",
"first-page": "718",
"journal-title": "Nature",
"key": "2023032213100791000_2023.03.17.23287398v1.32",
"volume": "595",
"year": "2021"
},
{
"DOI": "10.1016/j.celrep.2021.109869",
"article-title": "A potent bispecific nanobody protects hACE2 mice against SARS-CoV-2 infection via intranasal administration",
"doi-asserted-by": "crossref",
"first-page": "109869",
"journal-title": "Cell reports",
"key": "2023032213100791000_2023.03.17.23287398v1.33",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1007/s00253-021-11488-4",
"article-title": "Inhalation monoclonal antibody therapy: a new way to treat and manage respiratory infections",
"doi-asserted-by": "crossref",
"first-page": "6315",
"journal-title": "Applied microbiology and biotechnology",
"key": "2023032213100791000_2023.03.17.23287398v1.34",
"volume": "105",
"year": "2021"
},
{
"DOI": "10.3390/ijms20061379",
"doi-asserted-by": "crossref",
"key": "2023032213100791000_2023.03.17.23287398v1.35",
"unstructured": "E. Bequignon , C. Dhommée , C. Angely , L. Thomas , M. Bottier , E. Escudier , D. Isabey , A. Coste , B. Louis , J. F. Papon , V. Gouilleux-Gruart , FcRn-Dependent Transcytosis of Monoclonal Antibody in Human Nasal Epithelial Cells In Vitro: A Prerequisite for a New Delivery Route for Therapy? International journal of molecular sciences 20, (2019)."
},
{
"DOI": "10.1016/j.pharmthera.2021.108022",
"article-title": "Therapeutic antibodies - natural and pathological barriers and strategies to overcome them",
"doi-asserted-by": "crossref",
"first-page": "108022",
"journal-title": "Pharmacology & therapeutics",
"key": "2023032213100791000_2023.03.17.23287398v1.36",
"volume": "233",
"year": "2022"
},
{
"DOI": "10.1073/pnas.1208857109",
"doi-asserted-by": "publisher",
"key": "2023032213100791000_2023.03.17.23287398v1.37"
},
{
"DOI": "10.3389/fimmu.2021.653189",
"article-title": "A Rapid and Efficient Screening System for Neutralizing Antibodies and Its Application for SARS-CoV-2",
"doi-asserted-by": "crossref",
"first-page": "653189",
"journal-title": "Frontiers in immunology",
"key": "2023032213100791000_2023.03.17.23287398v1.38",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-26539-7",
"article-title": "Potent SARS-CoV-2 neutralizing antibodies with protective efficacy against newly emerged mutational variants",
"doi-asserted-by": "crossref",
"first-page": "6304",
"journal-title": "Nature communications",
"key": "2023032213100791000_2023.03.17.23287398v1.39",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2021.789905",
"article-title": "A Highly Conserved Peptide Vaccine Candidate Activates Both Humoral and Cellular Immunity Against SARS-CoV-2 Variant Strains",
"doi-asserted-by": "crossref",
"first-page": "789905",
"journal-title": "Frontiers in immunology",
"key": "2023032213100791000_2023.03.17.23287398v1.40",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2120219",
"article-title": "Resistance Mutations in SARS-CoV-2 Delta Variant after Sotrovimab Use",
"doi-asserted-by": "crossref",
"first-page": "1477",
"journal-title": "The New England journal of medicine",
"key": "2023032213100791000_2023.03.17.23287398v1.41",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41467-020-20789-7",
"article-title": "Molecular determinants and mechanism for antibody cocktail preventing SARS-CoV-2 escape",
"doi-asserted-by": "crossref",
"first-page": "469",
"journal-title": "Nature communications",
"key": "2023032213100791000_2023.03.17.23287398v1.42",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41467-022-30163-4",
"article-title": "Cumulative SARS-CoV-2 mutations and corresponding changes in immunity in an immunocompromised patient indicate viral evolution within the host",
"doi-asserted-by": "crossref",
"first-page": "2560",
"journal-title": "Nature communications",
"key": "2023032213100791000_2023.03.17.23287398v1.43",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1146/annurev-med-042420-113838",
"article-title": "SARS-CoV-2 Neutralizing Antibodies for COVID-19 Prevention and Treatment",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Annual review of medicine",
"key": "2023032213100791000_2023.03.17.23287398v1.44",
"volume": "73",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04661-w",
"doi-asserted-by": "publisher",
"key": "2023032213100791000_2023.03.17.23287398v1.45"
},
{
"DOI": "10.1038/s41467-022-32216-0",
"article-title": "An intranasal ASO therapeutic targeting SARS-CoV-2",
"doi-asserted-by": "crossref",
"first-page": "4503",
"journal-title": "Nature communications",
"key": "2023032213100791000_2023.03.17.23287398v1.46",
"volume": "13",
"year": "2022"
},
{
"article-title": "Inhalation drug delivery devices: technology update",
"first-page": "131",
"journal-title": "Medical devices (Auckland, N.Z.)",
"key": "2023032213100791000_2023.03.17.23287398v1.47",
"volume": "8",
"year": "2015"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"doi-asserted-by": "publisher",
"key": "2023032213100791000_2023.03.17.23287398v1.48"
},
{
"DOI": "10.1016/j.celrep.2022.110770",
"article-title": "Structures of Omicron spike complexes and implications for neutralizing antibody development",
"doi-asserted-by": "crossref",
"first-page": "110770",
"journal-title": "Cell reports",
"key": "2023032213100791000_2023.03.17.23287398v1.49",
"volume": "39",
"year": "2022"
},
{
"DOI": "10.1016/j.celrep.2021.110218",
"article-title": "The SARS-CoV-2 Lambda variant exhibits enhanced infectivity and immune resistance",
"doi-asserted-by": "crossref",
"first-page": "110218",
"journal-title": "Cell reports",
"key": "2023032213100791000_2023.03.17.23287398v1.50",
"volume": "38",
"year": "2022"
},
{
"DOI": "10.1038/s41467-021-25153-x",
"article-title": "Identification of potent human neutralizing antibodies against SARS-CoV-2 implications for development of therapeutics and prophylactics",
"doi-asserted-by": "crossref",
"first-page": "4887",
"journal-title": "Nature communications",
"key": "2023032213100791000_2023.03.17.23287398v1.51",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.jsb.2005.07.007",
"doi-asserted-by": "publisher",
"key": "2023032213100791000_2023.03.17.23287398v1.52"
},
{
"DOI": "10.1038/nmeth.4193",
"article-title": "MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy",
"doi-asserted-by": "crossref",
"first-page": "331",
"journal-title": "Nature Methods",
"key": "2023032213100791000_2023.03.17.23287398v1.53",
"volume": "14",
"year": "2017"
},
{
"DOI": "10.1038/nmeth.4169",
"article-title": "cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination",
"doi-asserted-by": "crossref",
"first-page": "290",
"journal-title": "Nature Methods",
"key": "2023032213100791000_2023.03.17.23287398v1.54",
"volume": "14",
"year": "2017"
},
{
"DOI": "10.1002/jcc.20084",
"doi-asserted-by": "publisher",
"key": "2023032213100791000_2023.03.17.23287398v1.55"
},
{
"DOI": "10.1038/nprot.2015.053",
"article-title": "The Phyre2 web portal for protein modeling, prediction and analysis",
"doi-asserted-by": "crossref",
"first-page": "845",
"journal-title": "Nature Protocols",
"key": "2023032213100791000_2023.03.17.23287398v1.56",
"volume": "10",
"year": "2015"
},
{
"DOI": "10.1107/S0907444910007493",
"doi-asserted-by": "publisher",
"key": "2023032213100791000_2023.03.17.23287398v1.57"
},
{
"DOI": "10.1107/S2059798319011471",
"article-title": "Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix",
"doi-asserted-by": "crossref",
"first-page": "861",
"journal-title": "Acta Crystallographica Section D",
"key": "2023032213100791000_2023.03.17.23287398v1.58",
"volume": "75",
"year": "2019"
},
{
"article-title": "An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques",
"first-page": "1",
"journal-title": "Nature communications",
"key": "2023032213100791000_2023.03.17.23287398v1.59",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-00405-5",
"doi-asserted-by": "crossref",
"key": "2023032213100791000_2023.03.17.23287398v1.60",
"unstructured": "Y. N. Zhang , X. D. Li , Z. R. Zhang , H. Q. Zhang , B. Zhang , A mouse model for SARS-CoV-2 infection by exogenous delivery of hACE2 using alphavirus replicon particles. Cell Research, 1–3 (2020)."
}
],
"reference-count": 60,
"references-count": 60,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2023.03.17.23287398"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "A first-in-human clinical study of an intranasal spray of a cocktail containing two synergetic antibodies neutralizes Omicron BA.4/5",
"type": "posted-content"
}