The Effect of Montelukast Treatment on Elderly Patients Diagnosed with COVID-19
et al., Genel Tıp Dergisi, doi:10.54005/geneltip.1352153, Aug 2024
32nd treatment shown to reduce risk in
November 2021, now with p = 0.0041 from 9 studies.
Lower risk for hospitalization and cases.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
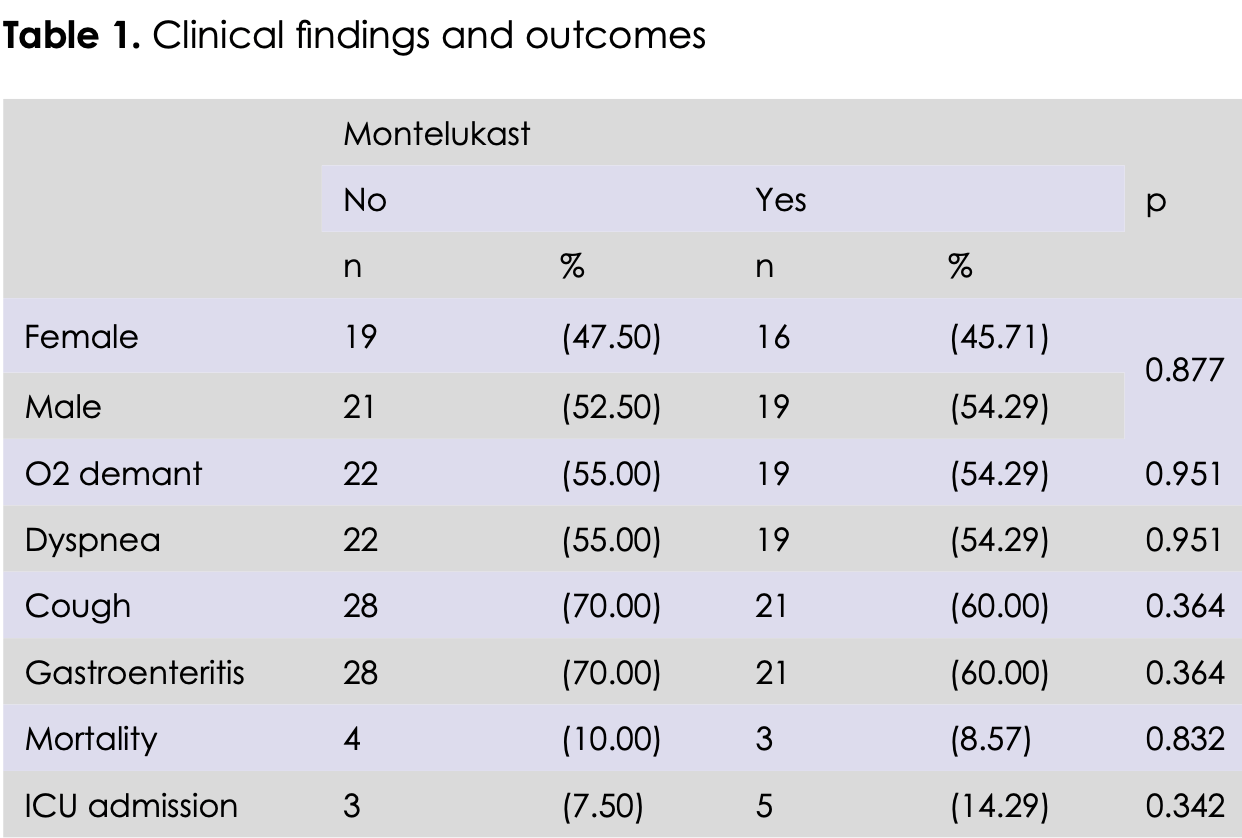
Retrospective 75 hospitalized COVID-19 patients over 60 in Turkey showing no significant differences with montelukast treatment.
This study is excluded in the after exclusion results of meta-analysis:
unadjusted results with minimal group details.
|
risk of death, 14.3% lower, RR 0.86, p = 1.00, treatment 3 of 35 (8.6%), control 4 of 40 (10.0%), NNT 70.
|
|
risk of ICU admission, 90.5% higher, RR 1.90, p = 0.46, treatment 5 of 35 (14.3%), control 3 of 40 (7.5%).
|
|
hospitalization time, 3.4% lower, relative time 0.97, p = 0.81, treatment mean 10.51 (±5.44) n=35, control mean 10.88 (±7.24) n=40.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Zengin et al., 5 Aug 2024, retrospective, Turkey, peer-reviewed, 9 authors, study period September 2021 - December 2022.
Contact: oguzhanzengin91@gmail.com, geneltip@selcuk.edu.tr.
The Effect of Montelukast Treatment on Elderly Patients Diagnosed with COVID-19
Genel Tıp Dergisi, doi:10.54005/geneltip.1352153
Objective: The clinical course in COVID-19 patients can vary from asymptomatic cases to acute respiratory distress syndrome (ARDS), multi-organ dysfunction and respiratory failure. Clinical progression is thought to be mainly due to the release of proinflammatory cytokines. The most common symptoms are shortness of breath, fever, malaise, and cough. Montelukast, which is used in the treatment of seasonal allergic rhinitis and asthma, started to be used in Covid-19 infection due to its anti-inflammatory and cytokine release-reducing effect. There are studies in the literature showing that montelukast treatment is beneficial in the treatment of COVID-19. However, there are not enough studies evaluating the efficacy of montelukast treatment in elderly patients. The aim of our study is to evaluate the clinical and laboratory efficacy of montelukast treatment in patients aged 60 and over in COVID-19 disease, and to indicate the differences from the studies in the literature. Method: Our research was planned as a retrospective, single-center, observational study. The medical records of 75 COVID-19 patients aged 60 and over who were hospitalized in the internal medicine clinic of Ankara Bilkent City Hospital between September 2021 and December 2022 were included. Results: Clinical findings and results were compared between the patients who received montelukast and the control group. There was no statistically significant difference between two groups in terms of cough, dyspnea, gastroenteritis and oxygen theraphy requirement. There wa no significant difference between the groups in terms of the need for intensive care unit admission and mortality. The length of hospital stay was compared in both groups, it was 10.88±7.24 days in the control group and 10.51±5.44 days in the montelukast group, and there was no statistically significant difference between the groups. The laboratory parameters of the patients in both groups were compared. The neutrophil count and leukocyte count measured before hospitalization were found significantly lower in the patient group receiving montelukast (p<0.05). No significant difference was found in other laboratory parameters.
Conclusion: Although montelukast treatment has positive effects on prognosis in COVID 19 disease in the literature, a similar effect was not observed in the population aged 60 and over in our study. We did not find a beneficial effect of short-term montelukast use on prognosis in COVID-19 patients aged 60 and over. We thought that this was due to the low efficacy of montelukast in the elderly population. Our study is a rare study in that it examines montelukast treatment in the geriatric population with COVID-19.
Ethical Approval Ethical approval for the study was granted by the Ethics Committee of Ankara City Hospital (Date: 12/04/2023, Number: E2-23-3911).
Authorship Contributions All of the authors declare that they have all participated in the design, execution, and analysis of the paper and that they have approved the final version.
Conflict of Interests The authors have no conflicts of interest to declare.
Financial Disclosure The authors declared that this study had received no financial support.
References
Aigner, The leukotriene receptor antagonist montelukast as a potential COVID-19 therapeutic, Frontiers in molecular biosciences
Bisgaard, Flores-Nunez, Goh, Azimi, Halkas et al., Study of montelukast for the treatment of respiratory symptoms of post-respiratory syncytial virus bronchiolitis in children, Am J Respir Critical Care Med
Bozek, Winterstein, Montelukast's ability to fight COVID-19 infection, Journal of Asthma
Bäck, Leukotriene signaling in atherosclerosis and ischemia, Cardiovascular drugs and therapy
Cbs, York, Though Not FDA Approved, Off-Label Singulair Showing Promise As Coronavirus Treatment, Say Doctors
Chen, Wu, Guo, Cao, Huang et al., Clinical and immunological features of severe and moderate coronavirus disease 2019, J Clin Invest, doi:10.1172/JCI137244
Chen, Zhang, Pan, Effect of Montelukast on Bronchopulmonary Dysplasia (BPD) and Related Mechanisms. Medical science monitor, int Med J Exp Clin Res
Columbo, Asthma in the elderly: a double-blind, placebocontrolled study of the effect of montelukast, Asthma research and practice
Davino-Chiovatto, Mc, Mackenzie, Santos-Dias, Almeida-Oliveira et al., Montelukast, leukotriene inhibitor, reduces LPS-induced acute lung inflammation and human neutrophil activation, Arch Bronconeumol, doi:10.1016/j.arbres.2019.05.003
Erfen, Akbay Çetin, Therapeutic and Preventive Effects of Piperine and its Combination with Curcumin as a Bioenhancer Against Aluminum-Induced Damage in the Astrocyte Cells, Neurotoxicity Research
Fidan, Aydoğdu, As a potential treatment of COVID-19: Montelukast, Medical hypotheses
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med, doi:10.1056/nejmoa2002032
Horiguchi, Tachikawa, Kondo, Comparative evaluation of the leukotriene receptor antagonist pranlukast versus the steroid inhalant fluticasone in the therapy of aged patients with mild bronchial asthma, Arzneimittelforschung
Kerget, Buğra, Effect of montelukast therapy on clinical course, pulmonary function, and mortality in patients with COVID-19, Journal of Medical Virology
Khan, Montelukast in hospitalized patients diagnosed with COVID-19, Journal of Asthma
Korenblat, Effect of age on response to zafirlukast in patients with asthma in the Accolate Clinical Experience and Pharmacoepidemiology Trial (ACCEPT), Annals of Allergy, Asthma & Immunology
Mason, Pathogenesis of COVID-19 from a cell biology perspective, Eur Respir J, doi:10.1183/13993003.00607-2020
Mullol, Callejas, Méndez-Arancibia, Fuentes, Alobid et al., Montelukast reduces eosinophilic inflammation by inhibiting both epithelial cell cytokine secretion (GM-CSF, IL-6, IL-8) and eosinophil survival
Noor, Najmi, Bukhtiar, Effect of Montelukast on bradykinininduced contraction of isolated tracheal smooth muscle of guinea pig, Indian J Pharmacol
Pedersen, Ho, SARS-CoV-2: a storm is raging, J Clin Invest, doi:10.1172/JCI137647
Rodriguez-Morales, Cardona-Ospina, Gutierrez-Ocampo, Villamizar-Pena, Holguin-Rivera et al., Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis, Travel Med Infect Dis
Russmann, Iselin, Meier, Acute hepatitis associated with montelukast, J Hepatol
Sanghai, Tranmer, Taming the cytokine storm: repurposing montelukast for the attenuation and prophylaxis of severe COVID-19 symptoms, Drug discovery today
Sarzi-Puttini, Sirotti, Marotto, Ardizzone, Rizzardini, COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome?, Clin Exp Rheumatol
Scicolone, Safety and efficacy of montelukast as adjunctive therapy for treatment of asthma in elderly patients, Clinical Interventions in Aging
Sánchez, Buitrago, Effect of Montelukast 10 mg in Elderly Patients with Mild and Moderate Asthma Compared with Young Adults. Results of a Cohort Study, Open Respir Med J, doi:10.2174/1874306401812010067
Zhang, Dong, Cao, Yuan, Yang et al., Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China, Allergy
DOI record:
{
"DOI": "10.54005/geneltip.1352153",
"ISSN": [
"2602-3741"
],
"URL": "http://dx.doi.org/10.54005/geneltip.1352153",
"abstract": "<jats:p xml:lang=\"en\">Abstract\r\n\r\nBackground/Aims:\r\n\r\nThe clinical course in COVID-19 patients can vary from asymptomatic cases to acute respiratory distress syndrome (ARDS), respiratory failure and multiorgan dysfunction. Clinical progression is thought to be mainly due to the release of proinflammatory cytokines. The most common symptoms are fever, cough, malaise, and shortness of breath. Montelukast, which is used in the treatment of seasonal allergic rhinitis and asthma, has brought its use in COVID-19 infection due to its anti-inflammatory and cytokine secretion-reducing effect. There are many studies in the literature that montelukast treatment has a positive effect on the prognosis and mortality of COVID-19. However, there are not enough studies evaluating the efficacy of montelukast treatment in elderly patients. The aim of our study is to evaluate the clinical and laboratory efficacy of montelukast treatment in patients aged 60 and over in COVID-19 disease, and to indicate the differences from the studies in the literature.\r\n\r\nMethods\r\n\r\nOur research was planned as a retrospective, single-center, observational study. The medical records of 75 COVID-19 patients aged 60 and over who were hospitalized in the internal medicine clinic of Ankara Bilkent City Hospital between September 2021 and December 2022 were included. Diagnosis of COVID-19 was confirmed with a reverse transcription polymerase chain reaction (RT-PCR) test from nasopharyngeal swab.\r\n\r\nResults\r\n\r\nClinical findings and results were compared between the patients who received montelukast and the control group. There was no statistically significant difference between the two groups in terms of cough, dyspnea, gastroenteritis and oxygen theraphy requirement. There is no significant difference between the two groups in terms of the need for intensive care unit admission and mortality. The length of hospital stay was compared in both groups, it was 10.88±7.24 days in the control group and 10.51±5.44 days in the montelukast group, and there was no statistically significant difference between the groups. The laboratory parameters of the patients in both groups were compared. The neutrophil count and leukocyte count measured before hospitalization were found to be significantly lower in the patient group receiving montelukast (p=0.022, p=0.016). No significant difference was found in other laboratory parameters.\r\n\r\nConclusions\r\n\r\nAlthough montelukast treatment has positive effects on prognosis in COVID-19 disease in the literature, a similar effect was not observed in the population aged 60 and over in our study. We did not find the positive effect of short-term montelukast treatment on the prognosis of patients aged 60 years and older who were hospitalized due to COVID-19. We thought that this was due to the low efficacy of montelukast in the elderly population. Our study is one of the first to examine montelukast therapy in the geriatric population with COVID-19.</jats:p>",
"accepted": {
"date-parts": [
[
2024,
8,
5
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6575-4450",
"affiliation": [
{
"name": "SAĞLIK BİLİMLERİ ÜNİVERSİTESİ, ANKARA ŞEHİR SAĞLIK UYGULAMA VE ARAŞTIRMA MERKEZİ, DAHİLİ TIP BİLİMLERİ BÖLÜMÜ"
}
],
"authenticated-orcid": true,
"family": "Zengin",
"given": "Oğuzhan",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-7379-8330",
"affiliation": [
{
"name": "SAĞLIK BİLİMLERİ ÜNİVERSİTESİ, ANKARA ŞEHİR SAĞLIK UYGULAMA VE ARAŞTIRMA MERKEZİ, DAHİLİ TIP BİLİMLERİ BÖLÜMÜ"
}
],
"authenticated-orcid": true,
"family": "Aytekin",
"given": "Öztuğ",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8396-4152",
"affiliation": [
{
"name": "SAĞLIK BİLİMLERİ ÜNİVERSİTESİ, ANKARA ŞEHİR SAĞLIK UYGULAMA VE ARAŞTIRMA MERKEZİ, DAHİLİ TIP BİLİMLERİ BÖLÜMÜ"
}
],
"authenticated-orcid": true,
"family": "Doğru",
"given": "Mustafa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6082-8323",
"affiliation": [
{
"name": "SAĞLIK BİLİMLERİ ÜNİVERSİTESİ, ANKARA ŞEHİR SAĞLIK UYGULAMA VE ARAŞTIRMA MERKEZİ, DAHİLİ TIP BİLİMLERİ BÖLÜMÜ"
}
],
"authenticated-orcid": true,
"family": "Göre",
"given": "Burak",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3539-8875",
"affiliation": [
{
"name": "SAĞLIK BİLİMLERİ ÜNİVERSİTESİ, ANKARA ŞEHİR SAĞLIK UYGULAMA VE ARAŞTIRMA MERKEZİ, DAHİLİ TIP BİLİMLERİ BÖLÜMÜ"
}
],
"authenticated-orcid": true,
"family": "Sözen",
"given": "Emine Sena",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7252-1292",
"affiliation": [
{
"name": "SAĞLIK BİLİMLERİ ÜNİVERSİTESİ, ANKARA ŞEHİR SAĞLIK UYGULAMA VE ARAŞTIRMA MERKEZİ, DAHİLİ TIP BİLİMLERİ BÖLÜMÜ"
}
],
"authenticated-orcid": true,
"family": "Evli",
"given": "Merve",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4552-0387",
"affiliation": [
{
"name": "SAĞLIK BİLİMLERİ ÜNİVERSİTESİ, ANKARA ŞEHİR SAĞLIK UYGULAMA VE ARAŞTIRMA MERKEZİ, DAHİLİ TIP BİLİMLERİ BÖLÜMÜ"
}
],
"authenticated-orcid": true,
"family": "Şahiner",
"given": "Enes Seyda",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8717-3013",
"affiliation": [
{
"name": "SAĞLIK BİLİMLERİ ÜNİVERSİTESİ, ANKARA ŞEHİR SAĞLIK UYGULAMA VE ARAŞTIRMA MERKEZİ, DAHİLİ TIP BİLİMLERİ BÖLÜMÜ"
}
],
"authenticated-orcid": true,
"family": "İnan",
"given": "Osman",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2858-6229",
"affiliation": [
{
"name": "SAĞLIK BİLİMLERİ ÜNİVERSİTESİ, ANKARA ŞEHİR SAĞLIK UYGULAMA VE ARAŞTIRMA MERKEZİ, DAHİLİ TIP BİLİMLERİ BÖLÜMÜ"
}
],
"authenticated-orcid": true,
"family": "Ateş",
"given": "İhsan",
"sequence": "additional"
}
],
"container-title": "Genel Tıp Dergisi",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
8,
25
]
],
"date-time": "2024-08-25T19:01:17Z",
"timestamp": 1724612477000
},
"deposited": {
"date-parts": [
[
2024,
8,
28
]
],
"date-time": "2024-08-28T22:46:34Z",
"timestamp": 1724885194000
},
"indexed": {
"date-parts": [
[
2024,
8,
29
]
],
"date-time": "2024-08-29T00:25:36Z",
"timestamp": 1724891136393
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
8,
5
]
]
},
"member": "9978",
"original-title": [],
"prefix": "10.54005",
"published": {
"date-parts": [
[
2024,
8,
5
]
]
},
"published-online": {
"date-parts": [
[
2024,
8,
5
]
]
},
"publisher": "Selcuk University",
"reference": [
{
"DOI": "10.1080/02770903.2021.1881967",
"doi-asserted-by": "crossref",
"key": "ref1",
"unstructured": "Khan, Ahsan R., et al. \"Montelukast in hospitalized patients diagnosed with COVID-19.\" Journal of Asthma 59.4 (2022): 780-786."
},
{
"DOI": "10.1016/j.mehy.2020.109828",
"doi-asserted-by": "crossref",
"key": "ref2",
"unstructured": "Fidan, Cihan, and Ayşe Aydoğdu. \"As a potential treatment of COVID-19: Montelukast.\" Medical hypotheses 142 (2020): 109828."
},
{
"DOI": "10.1016/j.tmaid.2020.101623",
"doi-asserted-by": "crossref",
"key": "ref3",
"unstructured": "Rodriguez-Morales AJ, Cardona-Ospina JA, Gutierrez-Ocampo E, Villamizar-Pena R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 2020:101623. PubMed PMID: 32179124. Epub 2020/03/18. eng."
},
{
"DOI": "10.1111/all.14238",
"doi-asserted-by": "crossref",
"key": "ref4",
"unstructured": "Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020. PubMed PMID: 32077115. Epub 2020/02/23. eng."
},
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "crossref",
"key": "ref5",
"unstructured": "Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L, Hui DSC, China Medical Treatment Expert Group for Covid-19, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/nejmoa2002032."
},
{
"DOI": "10.1172/JCI137244",
"doi-asserted-by": "crossref",
"key": "ref6",
"unstructured": "Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620– 2629. doi:10.1172/JCI137244."
},
{
"DOI": "10.1172/JCI137647",
"doi-asserted-by": "crossref",
"key": "ref7",
"unstructured": "Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130(5):2202–2205. doi:10.1172/ JCI137647."
},
{
"DOI": "10.12659/MSM.912774",
"doi-asserted-by": "crossref",
"key": "#cr-split#-ref8.1",
"unstructured": "Chen X, Zhang X, Pan J. Effect of Montelukast on Bronchopulmonary Dysplasia (BPD) and Related Mechanisms. Medical science monitor : int Med J Exp Clin Res 2019 Mar 13"
},
{
"key": "#cr-split#-ref8.2",
"unstructured": "25: 1886-93. PubMed PMID: 30862773. Pubmed Central PMCID: PMC6427930. Epub 2019/03/14. eng."
},
{
"DOI": "10.55563/clinexprheumatol/xcdary",
"doi-asserted-by": "crossref",
"key": "ref9",
"unstructured": "Sarzi-Puttini P, Giorgi V, Sirotti S, Marotto D, Ardizzone S, Rizzardini G, et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol 2020;38(2):337–42. PubMed PMID: 32202240. Epub 2020/03/2eng."
},
{
"DOI": "10.21203/rs.3.rs-1959715/v1",
"doi-asserted-by": "crossref",
"key": "ref10",
"unstructured": "Erfen, Şebnem, and Esin Akbay Çetin. \"Therapeutic and Preventive Effects of Piperine and its Combination with Curcumin as a Bioenhancer Against Aluminum-Induced Damage in the Astrocyte Cells.\" Neurotoxicity Research (2022): 1-19."
},
{
"key": "ref11",
"unstructured": "Mullol J, Callejas FB, Méndez-Arancibia E, Fuentes M, Alobid I, Martinez-Anton A, Valero A, Picado C, Roca-Ferrer J. Montelukast reduces eosinophilic inflammation by inhibiting both epithelial cell cytokine secretion (GM-CSF, IL-6, IL-8) and eosinophil survival."
},
{
"DOI": "10.1183/13993003.00607-2020",
"doi-asserted-by": "crossref",
"key": "ref12",
"unstructured": "Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55(4):2000607. doi:10.1183/13993003.00607-2020."
},
{
"DOI": "10.1164/rccm.200706-910OC",
"doi-asserted-by": "crossref",
"key": "ref13",
"unstructured": "Bisgaard H, Flores-Nunez A, Goh A, Azimi P, Halkas A, Malice MP, et al. Study of montelukast for the treatment of respiratory symptoms of post-respiratory syncytial virus bronchiolitis in children. Am J Respir Critical Care Med 2008;178(8):854–60. PubMed PMID: 18583576. Epub 2008/06/28. eng."
},
{
"DOI": "10.4103/0253-7613.83119",
"doi-asserted-by": "crossref",
"key": "ref14",
"unstructured": "Noor A, Najmi MH, Bukhtiar S. Effect of Montelukast on bradykinin-induced contraction of isolated tracheal smooth muscle of guinea pig. Indian J Pharmacol 2011;43(4):445–9. PubMed PMID: 21845003. Pubmed Central PMCID: PMC3153711. Epub 2011/08/17. eng."
},
{
"DOI": "10.1016/j.arbres.2019.05.003",
"doi-asserted-by": "crossref",
"key": "ref15",
"unstructured": "Davino-Chiovatto JE, Oliveira-Junior MC, MacKenzie B, Santos-Dias A, Almeida-Oliveira AR, Aquino-Junior JCJ, Br ito AA, R igonato-Oliveira NC, Damaceno-Rodrigues NR, Oliveira APL, et al. Montelukast, leukotriene inhibitor, reduces LPS-induced acute lung inflammation and human neutrophil activation. Arch Bronconeumol. 2019;55(11):573–580. doi:10.1016/j.arbres.2019.05.003"
},
{
"key": "ref16",
"unstructured": "CBS New York. Though Not FDA Approved, Off-Label Singulair Showing Promise As Coronavirus Treatment, Say Doctors. 2020."
},
{
"DOI": "10.3389/fmolb.2020.610132",
"doi-asserted-by": "crossref",
"key": "ref17",
"unstructured": "Aigner, Ludwig, et al. \"The leukotriene receptor antagonist montelukast as a potential COVID-19 therapeutic.\" Frontiers in molecular biosciences 7 (2020): 610132."
},
{
"DOI": "10.1002/jmv.27552",
"doi-asserted-by": "crossref",
"key": "ref18",
"unstructured": "Kerget, Buğra, et al. \"Effect of montelukast therapy on clinical course, pulmonary function, and mortality in patients with COVID‐19.\" Journal of Medical Virology 94.5 (2022): 1950-1958."
},
{
"DOI": "10.1016/j.drudis.2020.09.013",
"doi-asserted-by": "crossref",
"key": "ref19",
"unstructured": "Sanghai, Nitesh, and Geoffrey K. Tranmer. \"Taming the cytokine storm: repurposing montelukast for the attenuation and prophylaxis of severe COVID-19 symptoms.\" Drug discovery today 25.12 (2020): 2076-2079."
},
{
"DOI": "10.1186/s40733-017-0031-4",
"doi-asserted-by": "crossref",
"key": "ref20",
"unstructured": "Columbo, Michele. \"Asthma in the elderly: a double-blind, placebo-controlled study of the effect of montelukast.\" Asthma research and practice 3 (2017): 1-4."
},
{
"DOI": "10.1080/02770903.2020.1786112",
"doi-asserted-by": "crossref",
"key": "ref21",
"unstructured": "Bozek, Andrzej, and Janne Winterstein. \"Montelukast’s ability to fight COVID-19 infection.\" Journal of Asthma 58.10 (2021): 1348-1349."
},
{
"DOI": "10.1016/S1081-1206(10)62759-7",
"doi-asserted-by": "crossref",
"key": "ref22",
"unstructured": "Korenblat, Phillip E., et al. \"Effect of age on response to zafirlukast in patients with asthma in the Accolate Clinical Experience and Pharmacoepidemiology Trial (ACCEPT).\" Annals of Allergy, Asthma & Immunology 84.2 (2000): 217-225."
},
{
"DOI": "10.1055/s-0031-1296588",
"doi-asserted-by": "crossref",
"key": "ref23",
"unstructured": "Horiguchi T, Tachikawa S, Kondo R, et al. Comparative evaluation of the leukotriene receptor antagonist pranlukast versus the steroid inhalant fluticasone in the therapy of aged patients with mild bronchial asthma. Arzneimittelforschung. 2007;57(2):87–91."
},
{
"DOI": "10.2147/CIA.S35977",
"doi-asserted-by": "crossref",
"key": "ref24",
"unstructured": "Scicolone, Nicola, et al. \"Safety and efficacy of montelukast as adjunctive therapy for treatment of asthma in elderly patients.\" Clinical Interventions in Aging (2013): 1329-1337."
},
{
"DOI": "10.1007/s10557-008-6140-9",
"doi-asserted-by": "crossref",
"key": "ref25",
"unstructured": "Bäck, Magnus. \"Leukotriene signaling in atherosclerosis and ischemia.\" Cardiovascular drugs and therapy 23 (2009): 41-48."
},
{
"DOI": "10.1016/S0168-8278(03)00085-0",
"doi-asserted-by": "crossref",
"key": "ref26",
"unstructured": "Russmann S, Iselin HU, Meier D, et al. Acute hepatitis associated with montelukast. J Hepatol. 2003;38(5):694–695"
},
{
"DOI": "10.2174/1874306401812010067",
"doi-asserted-by": "crossref",
"key": "ref27",
"unstructured": "Sánchez G, Buitrago D. Effect of Montelukast 10 mg in Elderly Patients with Mild and Moderate Asthma Compared with Young Adults. Results of a Cohort Study. Open Respir Med J. 2018;12:67-74. Published 2018 Nov 14. doi:10.2174/1874306401812010067"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "http://dergipark.org.tr/en/doi/10.54005/geneltip.1352153"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The Effect of Montelukast Treatment on Elderly Patients Diagnosed with COVID-19",
"type": "journal-article"
}
