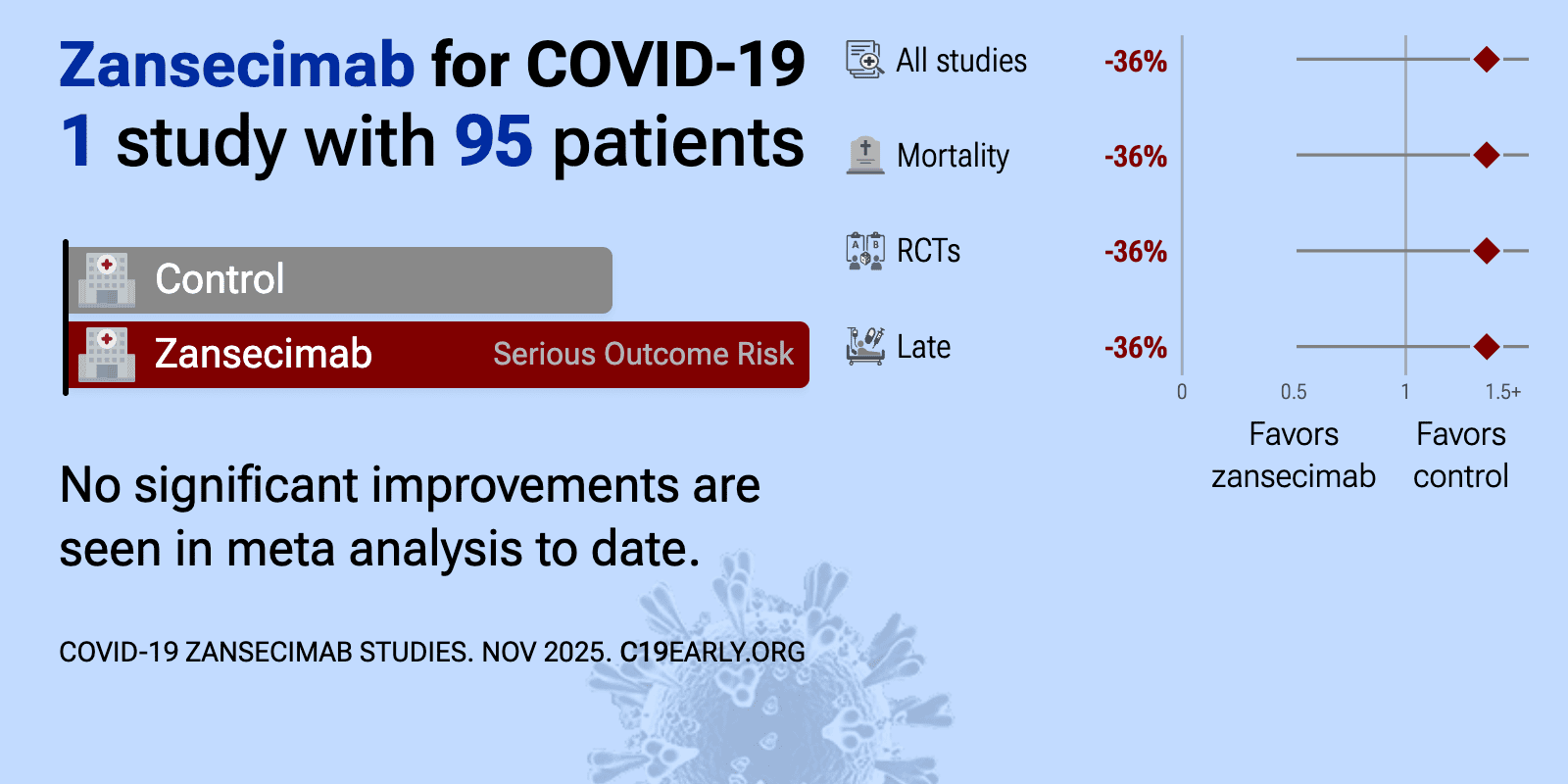
Zansecimab (LY3127804) is an intravenous monoclonal antibody that targets Angiopoietin-2 (Ang-2), a key protein in vascular inflammation.
Jan 31 2022 |
et al., Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine, doi:10.1177/11795484221119316 | Efficacy and Safety of LY3127804, an Anti-Angiopoietin-2 Antibody, in a Randomized, Double-Blind, Placebo-Controlled Clinical Trial in Patients Hospitalized with Pneumonia and Presumed or Confirmed COVID-19 |
| 36% higher mortality (p=0.58) and 70% higher combined mortality/intubation (p=0.28). RCT 95 hospitalized patients with COVID-19 pneumonia showing no significant differences with zansecimab (LY3127804, anti-Angiopoietin-2 antibody). | ||