ColdZyme® protects airway epithelia from infection with BA.4/5
et al., Respiratory Research, doi:10.1186/s12931-022-02223-2, Oct 2022
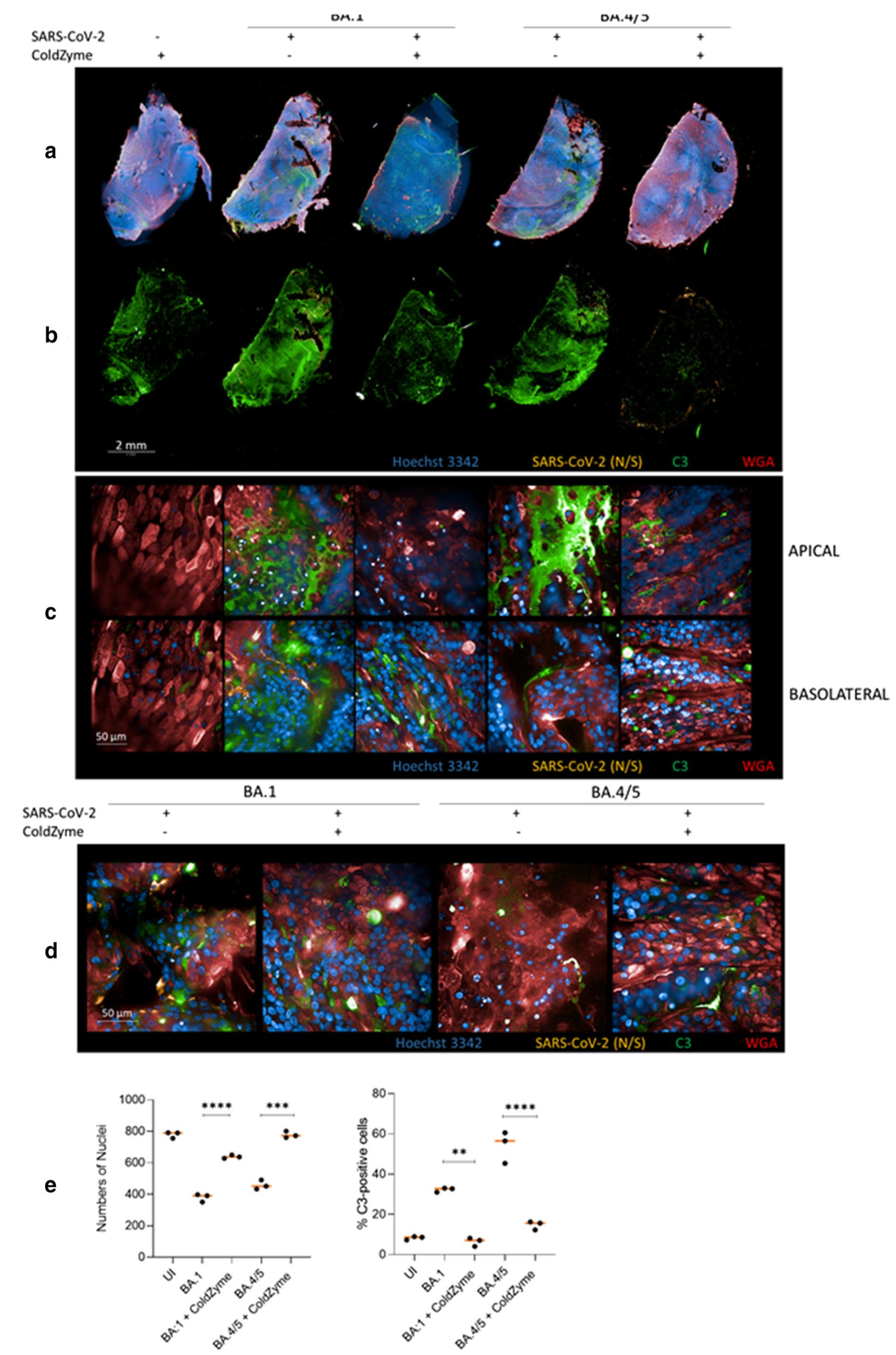
In vitro study showing that ColdZyme mouth spray protects human airway epithelial cells from infection with SARS-CoV-2 Omicron variants BA.1 and BA.4/5. Authors demonstrated that a single application of ColdZyme to fully differentiated human bronchial epithelial cells cultured at air-liquid interface prevented viral infection, maintained epithelial integrity, and blocked intracellular complement C3 activation. The spray, containing glycerol and cod trypsin, forms a physical barrier that traps and inactivates viruses. Application of ColdZyme significantly reduced viral loads in culture supernatants, with up to 2.8×10^9 copies/ml in untreated infected cultures compared to near-complete blockage in treated cultures. Transepithelial electrical resistance measurements confirmed that ColdZyme prevented virus-induced damage to tissue integrity. The protective effect was maintained for at least 3 days after a single application, suggesting potential utility as a prophylactic treatment against highly transmissible SARS-CoV-2 variants.
Zaderer et al., 31 Oct 2022, peer-reviewed, 6 authors.
Contact: wilfried.posch@i-med.ac.at, doris.wilflingseder@i-med.ac.at.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
ColdZyme® protects airway epithelia from infection with BA.4/5
Respiratory Research, doi:10.1186/s12931-022-02223-2
Vaccines against SARS-CoV-2 protect from critical or severe pathogenesis also against new variants of concern (VOCs) such as BA.4 and BA.5, but immediate interventions to avoid viral transmission and subsequent inflammatory reactions are needed. Here we applied the ColdZyme ® medical device mouth spray to fully differentiated, polarized human epithelium cultured at an air-liquid interphase (ALI). We found using VOCs BA.1 and BA.4/5 that this device effectively blocked respiratory tissue infection. While infection with these VOCs resulted in intracellular complement activation, thus enhanced inflammation, and drop of transepithelial resistance, these phenomena were prevented by a single administration of this medical device. Thus, ColdZyme ® mouth spray significantly shields epithelial integrity, hinders virus infection and blocks in a secondary effect intrinsic complement activation within airway cultures also in terms of the highly contagious VOCs BA.4/5. Crucially, our in vitro data suggest that ColdZyme ® mouth spray may have an impact to protect against SARS-CoV-2 transmission, also in case of the Omicron BA.1, BA.4 and BA.5 variants.
Author contributions VZ and SD performed experiments, prepared figures and analyzed data, RB-W acquired SARS-CoV-2 patient isolates, supported drafting the manuscript and substantially revised it, CL-F supported data interpretation, drafting and revising the manuscript, WP and DW designed the study, analyzed data and performed statistics, drafted the manuscript and included revisions, finalized the manuscript. All authors reviewed and approved the submitted version. All authors have agreed to being accountable for their contributions and for submitting an accurate and integer work.
Declarations Ethical approval and consent to participate Written informed consent was obtained from all donors of leftover nasopharyngeal/ oropharyngeal specimens and EDTA blood by the participating clinics. The Ethics Committee of the Medical University of Innsbruck (a copy is attached to the proposal, ECS1166/2020) approved the use of anonymized leftover specimens of COVID-19 patients for scientific purposes. All authors read and approved the final manuscript.
Competing interests The study was partly financially supported by Enzymatica AB. The funders had no influence on study design, analysis and data validation. The major part
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Afzali, Noris, Lambrecht, Kemper, The state of complement in COVID-19, Nat Rev Immunol, doi:10.1038/s41577-021-00665-1
Carreno, Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron, Nature, doi:10.1038/s41586-022-04399-5
Chandorkar, Fast-track development of an in vitro 3D lung/ immune cell model to study Aspergillus infections, Sci Rep, doi:10.1038/s41598-017-11271-4
Gralinski, Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis, MBio, doi:10.1128/mBio.01753-18
Gu, Crosstalk between TGF-beta 1 and complement activation augments epithelial injury in pulmonary fibrosis, Faseb J, doi:10.1096/fj.13-247650
Gudmundsdottir, Scheving, Lindberg, Stefansson, Inactivation of SARS-CoV-2 and HCoV-229E in vitro by ColdZyme(R) a medical device mouth spray against the common cold, J Med Virol, doi:10.1002/jmv.26554
Gudmundsdóttir, Hilmarsson, Stefansson, Potential use of Atlantic cod trypsin in biomedicine, Biomed Res Int, doi:10.1155/2013/749078
Liu, Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2, Nature, doi:10.1038/s41586-021-04388-0
Matsuyama, Enhanced isolation of SARS-CoV-2 by TMPRSS2expressing cells, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2002589117
Muik, Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera, Science, doi:10.1126/science.abn7591
Planas, Considerable escape of SARS-CoV-2 Omicron to antibody neutralization, Nature, doi:10.1038/s41586-021-04389-z
Posch, C5aR inhibition of nonimmune cells suppresses inflammation and maintains epithelial integrity in SARS-CoV-2-infected primary human airway epithelia, J Allergy Clin Immunol, doi:10.1016/j.jaci.2021.03.038
Posch, ColdZyme maintains integrity in SARS-CoV-2-infected airway epithelia, MBio, doi:10.1128/mBio.00904-21
Sandholt, Stefansson, Scheving, Gudmundsdottir, Biochemical characterization of a native group III trypsin ZT from Atlantic cod (Gadus morhua), Int J Biol Macromol, doi:10.1016/j.ijbiomac.2018.12.099
Sigal, Milder disease with Omicron: is it the virus or the pre-existing immunity?, Nat Rev Immunol, doi:10.1038/s41577-022-00678-4
Stefansson, Clarsund, A medical device forming a protective barrier that deactivates four major common cold viruses, Virol Res Rev, doi:10.15761/VRR.1000130
Tegally, Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa, Nat Med, doi:10.1038/s41591-022-01911-2
Thorne, Evolution of enhanced innate immune evasion by SARS-CoV-2, Nature, doi:10.1038/s41586-021-04352-y
Tuekprakhon, Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum, Cell, doi:10.1016/j.cell.2022.06.005
Xia, Neutralization and durability of 2 or 3 doses of the BNT162b2 vaccine against Omicron SARS-CoV-2, Cell Host Microbe, doi:10.1016/j.chom.2022.02.015
Yan, SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation, Sci Immunol, doi:10.1126/sciimmunol.abg0833
Zaderer, Hermann, Lass-Florl, Posch, Wilflingseder, Turning the world upside-down in cellulose for improved culturing and imaging of respiratory challenges within a human 3D model, Cells, doi:10.3390/cells8101292
DOI record:
{
"DOI": "10.1186/s12931-022-02223-2",
"ISSN": [
"1465-993X"
],
"URL": "http://dx.doi.org/10.1186/s12931-022-02223-2",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Vaccines against SARS-CoV-2 protect from critical or severe pathogenesis also against new variants of concern (VOCs) such as BA.4 and BA.5, but immediate interventions to avoid viral transmission and subsequent inflammatory reactions are needed. Here we applied the ColdZyme® medical device mouth spray to fully differentiated, polarized human epithelium cultured at an air-liquid interphase (ALI). We found using VOCs BA.1 and BA.4/5 that this device effectively blocked respiratory tissue infection. While infection with these VOCs resulted in intracellular complement activation, thus enhanced inflammation, and drop of transepithelial resistance, these phenomena were prevented by a single administration of this medical device. Thus, ColdZyme® mouth spray significantly shields epithelial integrity, hinders virus infection and blocks in a secondary effect intrinsic complement activation within airway cultures also in terms of the highly contagious VOCs BA.4/5. Crucially, our in vitro data suggest that ColdZyme® mouth spray may have an impact to protect against SARS-CoV-2 transmission, also in case of the Omicron BA.1, BA.4 and BA.5 variants.</jats:p>",
"alternative-id": [
"2223"
],
"article-number": "300",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "23 August 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "5 October 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "31 October 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Date",
"name": "change_date",
"order": 4,
"value": "24 January 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Type",
"name": "change_type",
"order": 5,
"value": "Correction"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Details",
"name": "change_details",
"order": 6,
"value": "A Correction to this paper has been published:"
},
{
"URL": "https://doi.org/10.1186/s12931-023-02326-4",
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Details",
"name": "change_details",
"order": 7,
"value": "https://doi.org/10.1186/s12931-023-02326-4"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethical approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Written informed consent was obtained from all donors of leftover nasopharyngeal/ oropharyngeal specimens and EDTA blood by the participating clinics. The Ethics Committee of the Medical University of Innsbruck (a copy is attached to the proposal, ECS1166/2020) approved the use of anonymized leftover specimens of COVID-19 patients for scientific purposes. All authors read and approved the final manuscript."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The study was partly financially supported by Enzymatica AB. The funders had no influence on study design, analysis and data validation. The major part of the study was supported by the FWF, Enzymatica only provided several materials."
}
],
"author": [
{
"affiliation": [],
"family": "Zaderer",
"given": "Viktoria",
"sequence": "first"
},
{
"affiliation": [],
"family": "Dichtl",
"given": "Stefanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bellmann Weiler",
"given": "Rosa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lass Flörl",
"given": "Cornelia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Posch",
"given": "Wilfried",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilflingseder",
"given": "Doris",
"sequence": "additional"
}
],
"container-title": "Respiratory Research",
"container-title-short": "Respir Res",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
10,
31
]
],
"date-time": "2022-10-31T19:02:54Z",
"timestamp": 1667242974000
},
"deposited": {
"date-parts": [
[
2023,
1,
25
]
],
"date-time": "2023-01-25T07:02:52Z",
"timestamp": 1674630172000
},
"funder": [
{
"DOI": "10.13039/501100002428",
"award": [
"P34070-B",
"P33510-B"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100002428",
"id-type": "DOI"
}
],
"name": "Austrian Science Fund"
}
],
"indexed": {
"date-parts": [
[
2025,
6,
9
]
],
"date-time": "2025-06-09T17:48:29Z",
"timestamp": 1749491309696,
"version": "3.37.3"
},
"is-referenced-by-count": 9,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
10,
31
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
31
]
],
"date-time": "2022-10-31T00:00:00Z",
"timestamp": 1667174400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
31
]
],
"date-time": "2022-10-31T00:00:00Z",
"timestamp": 1667174400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12931-022-02223-2.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12931-022-02223-2/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12931-022-02223-2.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2022,
10,
31
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
31
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1038/s41591-022-01911-2",
"author": "H Tegally",
"doi-asserted-by": "publisher",
"journal-title": "Nat Med",
"key": "2223_CR1",
"unstructured": "Tegally H, et al. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat Med. 2022. https://doi.org/10.1038/s41591-022-01911-2.",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2022.06.005",
"author": "A Tuekprakhon",
"doi-asserted-by": "publisher",
"first-page": "2422",
"journal-title": "Cell",
"key": "2223_CR2",
"unstructured": "Tuekprakhon A, et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185:2422-2433.e2413. https://doi.org/10.1016/j.cell.2022.06.005.",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04399-5",
"author": "JM Carreno",
"doi-asserted-by": "publisher",
"first-page": "682",
"journal-title": "Nature",
"key": "2223_CR3",
"unstructured": "Carreno JM, et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2022;602:682–8. https://doi.org/10.1038/s41586-022-04399-5.",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-04388-0",
"author": "L Liu",
"doi-asserted-by": "publisher",
"first-page": "676",
"journal-title": "Nature",
"key": "2223_CR4",
"unstructured": "Liu L, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602:676–81. https://doi.org/10.1038/s41586-021-04388-0.",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1126/science.abn7591",
"author": "A Muik",
"doi-asserted-by": "publisher",
"first-page": "678",
"journal-title": "Science",
"key": "2223_CR5",
"unstructured": "Muik A, et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science. 2022;375:678–80. https://doi.org/10.1126/science.abn7591.",
"volume": "375",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-04389-z",
"author": "D Planas",
"doi-asserted-by": "publisher",
"first-page": "671",
"journal-title": "Nature",
"key": "2223_CR6",
"unstructured": "Planas D, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602:671–5. https://doi.org/10.1038/s41586-021-04389-z.",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-04352-y",
"author": "LG Thorne",
"doi-asserted-by": "publisher",
"first-page": "487",
"journal-title": "Nature",
"key": "2223_CR7",
"unstructured": "Thorne LG, et al. Evolution of enhanced innate immune evasion by SARS-CoV-2. Nature. 2022;602:487–95. https://doi.org/10.1038/s41586-021-04352-y.",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1016/j.chom.2022.02.015",
"author": "H Xia",
"doi-asserted-by": "publisher",
"first-page": "485",
"journal-title": "Cell Host Microbe",
"key": "2223_CR8",
"unstructured": "Xia H, et al. Neutralization and durability of 2 or 3 doses of the BNT162b2 vaccine against Omicron SARS-CoV-2. Cell Host Microbe. 2022;30:485-488.e483. https://doi.org/10.1016/j.chom.2022.02.015.",
"volume": "30",
"year": "2022"
},
{
"DOI": "10.1128/mBio.00904-21",
"author": "W Posch",
"doi-asserted-by": "publisher",
"journal-title": "MBio.",
"key": "2223_CR9",
"unstructured": "Posch W, et al. ColdZyme maintains integrity in SARS-CoV-2-infected airway epithelia. MBio. 2021. https://doi.org/10.1128/mBio.00904-21.",
"year": "2021"
},
{
"DOI": "10.1016/j.ijbiomac.2018.12.099",
"author": "GB Sandholt",
"doi-asserted-by": "publisher",
"first-page": "847",
"journal-title": "Int J Biol Macromol",
"key": "2223_CR10",
"unstructured": "Sandholt GB, Stefansson B, Scheving R, Gudmundsdottir A. Biochemical characterization of a native group III trypsin ZT from Atlantic cod (Gadus morhua). Int J Biol Macromol. 2019;125:847–55. https://doi.org/10.1016/j.ijbiomac.2018.12.099.",
"volume": "125",
"year": "2019"
},
{
"DOI": "10.15761/VRR.1000130",
"author": "B Stefansson",
"doi-asserted-by": "publisher",
"journal-title": "Virol Res Rev.",
"key": "2223_CR11",
"unstructured": "Stefansson B, Clarsund GA. A medical device forming a protective barrier that deactivates four major common cold viruses. Virol Res Rev. 2017. https://doi.org/10.15761/VRR.1000130.",
"year": "2017"
},
{
"DOI": "10.1002/jmv.26554",
"author": "A Gudmundsdottir",
"doi-asserted-by": "publisher",
"journal-title": "J Med Virol",
"key": "2223_CR12",
"unstructured": "Gudmundsdottir A, Scheving R, Lindberg F, Stefansson B. Inactivation of SARS-CoV-2 and HCoV-229E in vitro by ColdZyme(R) a medical device mouth spray against the common cold. J Med Virol. 2020. https://doi.org/10.1002/jmv.26554.",
"year": "2020"
},
{
"DOI": "10.1016/j.jaci.2021.03.038",
"author": "W Posch",
"doi-asserted-by": "publisher",
"first-page": "2083",
"journal-title": "J Allergy Clin Immunol",
"key": "2223_CR13",
"unstructured": "Posch W, et al. C5aR inhibition of nonimmune cells suppresses inflammation and maintains epithelial integrity in SARS-CoV-2-infected primary human airway epithelia. J Allergy Clin Immunol. 2021;147:2083-2097.e2086. https://doi.org/10.1016/j.jaci.2021.03.038.",
"volume": "147",
"year": "2021"
},
{
"DOI": "10.1038/s41598-017-11271-4",
"author": "P Chandorkar",
"doi-asserted-by": "publisher",
"first-page": "11644",
"journal-title": "Sci Rep",
"key": "2223_CR14",
"unstructured": "Chandorkar P, et al. Fast-track development of an in vitro 3D lung/immune cell model to study Aspergillus infections. Sci Rep. 2017;7:11644. https://doi.org/10.1038/s41598-017-11271-4.",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.3390/cells8101292",
"author": "V Zaderer",
"doi-asserted-by": "publisher",
"journal-title": "Cells",
"key": "2223_CR15",
"unstructured": "Zaderer V, Hermann M, Lass-Florl C, Posch W, Wilflingseder D. Turning the world upside-down in cellulose for improved culturing and imaging of respiratory challenges within a human 3D model. Cells. 2019. https://doi.org/10.3390/cells8101292.",
"year": "2019"
},
{
"DOI": "10.1073/pnas.2002589117",
"author": "S Matsuyama",
"doi-asserted-by": "publisher",
"first-page": "7001",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "2223_CR16",
"unstructured": "Matsuyama S, et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A. 2020;117:7001–3. https://doi.org/10.1073/pnas.2002589117.",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1155/2013/749078",
"author": "A Gudmundsdóttir",
"doi-asserted-by": "publisher",
"first-page": "749078",
"journal-title": "Biomed Res Int",
"key": "2223_CR17",
"unstructured": "Gudmundsdóttir A, Hilmarsson H, Stefansson B. Potential use of Atlantic cod trypsin in biomedicine. Biomed Res Int. 2013;2013:749078. https://doi.org/10.1155/2013/749078. Epub 2013 Feb 28. PMID: 23555095; PMCID: PMC3600245.",
"volume": "2013",
"year": "2013"
},
{
"DOI": "10.1038/s41577-021-00665-1",
"author": "B Afzali",
"doi-asserted-by": "publisher",
"first-page": "77",
"journal-title": "Nat Rev Immunol",
"key": "2223_CR18",
"unstructured": "Afzali B, Noris M, Lambrecht BN, Kemper C. The state of complement in COVID-19. Nat Rev Immunol. 2022;22:77–84. https://doi.org/10.1038/s41577-021-00665-1.",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1126/sciimmunol.abg0833",
"author": "B Yan",
"doi-asserted-by": "publisher",
"journal-title": "Sci Immunol.",
"key": "2223_CR19",
"unstructured": "Yan B, et al. SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation. Sci Immunol. 2021. https://doi.org/10.1126/sciimmunol.abg0833.",
"year": "2021"
},
{
"DOI": "10.1096/fj.13-247650",
"author": "HM Gu",
"doi-asserted-by": "publisher",
"first-page": "4223",
"journal-title": "Faseb J",
"key": "2223_CR20",
"unstructured": "Gu HM, et al. Crosstalk between TGF-beta 1 and complement activation augments epithelial injury in pulmonary fibrosis. Faseb J. 2014;28:4223–34. https://doi.org/10.1096/fj.13-247650.",
"volume": "28",
"year": "2014"
},
{
"DOI": "10.1128/mBio.01753-18",
"author": "LE Gralinski",
"doi-asserted-by": "publisher",
"journal-title": "MBio.",
"key": "2223_CR21",
"unstructured": "Gralinski LE, et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio. 2018. https://doi.org/10.1128/mBio.01753-18.",
"year": "2018"
},
{
"DOI": "10.1038/s41577-022-00678-4",
"author": "A Sigal",
"doi-asserted-by": "publisher",
"first-page": "69",
"journal-title": "Nat Rev Immunol",
"key": "2223_CR22",
"unstructured": "Sigal A. Milder disease with Omicron: is it the virus or the pre-existing immunity? Nat Rev Immunol. 2022;22:69–71. https://doi.org/10.1038/s41577-022-00678-4.",
"volume": "22",
"year": "2022"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-022-02223-2"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "ColdZyme® protects airway epithelia from infection with BA.4/5",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"updated-by": [
{
"DOI": "10.1186/s12931-023-02326-4",
"label": "Correction",
"source": "publisher",
"type": "correction",
"updated": {
"date-parts": [
[
2023,
1,
24
]
],
"date-time": "2023-01-24T00:00:00Z",
"timestamp": 1674518400000
}
}
],
"volume": "23"
}