Dynamic Model of Andrographolide Therapy for COVID-19
et al., 2023 IEEE 5th Eurasia Conference on Biomedical Engineering, Healthcare and Sustainability, doi:10.3390/engproc2023055081, Dec 2023
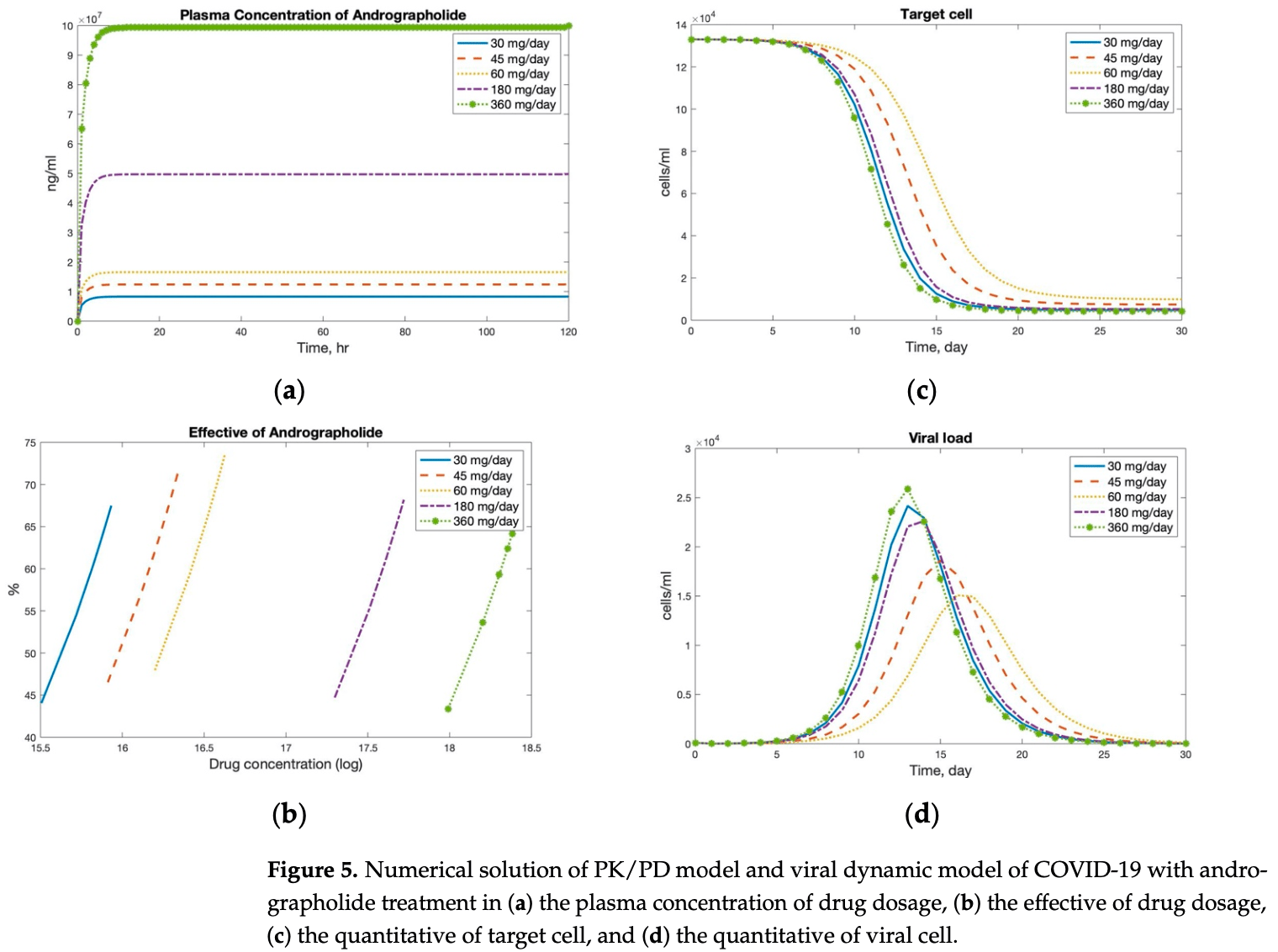
Pharmacokinetic and pharmacodynamic modeling of andrographolide for COVID-19. The model suggests that a dose of 60mg per day of andrographolide is optimal for reducing viral load and infection. The model incorporates the diffusion of the drug in plasma and tissues over time and its inhibitory impact on the viral life cycle.
Yarnvitayalert et al., 21 Dec 2023, Thailand, peer-reviewed, 2 authors.
Contact: teerapol.sal@kmutt.ac.th (corresponding author), panittavee.n@mail.kmutt.ac.th.
Dynamic Model of Andrographolide Therapy for COVID-19
doi:10.3390/engproc2023055081
Andrographis paniculate extract (APE) has been used as a Thailand traditional medicine owing to andrographolide which is effective for the viral clearance and prevention of disease progression. Several viral infections have been treated with APE, including SARS-CoV-2. The recommended dosage is 180 mg three times a day. We constructed a mathematical viral dynamic model of the SARS-CoV-2 model with andrographolide therapy by different doses. The pharmacokinetic/pharmacodynamic (PK/PD) model reduces the duration of viral clearance with a dose of 60 mg per day. Moreover, APE improved the therapeutic efficacy of COVID-19 therapy.
Conflicts of Interest: The authors declare no conflict of interest.
References
Dodds, Krishna, Goncalves, Rayner, Model-informed drug repurposing: Viral kinetic modelling to prioritize rational drug combinations for COVID-19, Br. J. Clin. Pharmacol, doi:10.1111/bcp.14486
Ducharme, Braga, Introduction to pharmacokinetic and pharmacodynamic models and analyses
Gonçalves, Bertrand, Ke, Comets, De Lamballerie et al., Timing of antiviral treatment initiation is critical to reduce SARS-CoV-2 viral load, CPT Pharmacomet. Syst. Pharmacol, doi:10.1002/psp4.12543
Kulichenko, Kireyeva, Malyshkina, Wikman, A randomized, controlled study of Kan Jang versus amantadine in the treatment of influenza in Volgograd, J. Herb. Pharmacother, doi:10.1080/J157v03n01_04
Panossian, Hovhannisyan, Mamikonyan, Abrahamian, Hambardzumyan et al., Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human, Phytomedicine, doi:10.1016/S0944-7113(00)80054-9
Sa-Ngiamsuntorn, Suksatu, Pewkliang, Thongsri, Kanjanasirirat et al., Anti-SARS-CoV-2 activity of andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives, J. Nat. Prod, doi:10.1021/acs.jnatprod.0c01324
Saxena, Singh, Kumar, Yadav, Negi et al., A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmCold) in patients with uncomplicated upper respiratory tract infection, Phytomedicine, doi:10.1016/j.phymed.2009.12.001
Thamlikitkul, Dechatiwongse, Theerapong, Chantrakul, Boonroj et al., Efficacy of Andrographis paniculata, Nees for pharyngotonsillistis in adults, J. Med. Assoc
Wanaratna, Leethong, Inchai, Chueawiang, Sriraksa et al., Efficacy and safety of Andrographis paniculata extract in patients with mild COVID-19: A randomized controlled trial, Arch. Intern. Med. Res, doi:10.26502/aimr.0125
DOI record:
{
"DOI": "10.3390/engproc2023055081",
"URL": "http://dx.doi.org/10.3390/engproc2023055081",
"alternative-id": [
"engproc2023055081"
],
"author": [
{
"ORCID": "http://orcid.org/0009-0003-4538-7608",
"affiliation": [
{
"name": "Department of Mathematics, Faculty of Science, King Mongkut’s University of Technology Thonburi, Bangkok 10140, Thailand"
}
],
"authenticated-orcid": false,
"family": "Yarnvitayalert",
"given": "Panittavee",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Mathematics, Faculty of Science, King Mongkut’s University of Technology Thonburi, Bangkok 10140, Thailand"
}
],
"family": "Saleewong",
"given": "Teerapol",
"sequence": "additional"
}
],
"container-title": "2023 IEEE 5th Eurasia Conference on Biomedical Engineering, Healthcare and Sustainability",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
12,
21
]
],
"date-time": "2023-12-21T10:15:15Z",
"timestamp": 1703153715000
},
"deposited": {
"date-parts": [
[
2023,
12,
21
]
],
"date-time": "2023-12-21T10:15:54Z",
"timestamp": 1703153754000
},
"event": "Eurasia Conference on Biomedical Engineering, Healthcare and Sustainability",
"indexed": {
"date-parts": [
[
2023,
12,
22
]
],
"date-time": "2023-12-22T00:16:10Z",
"timestamp": 1703204170363
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
12,
21
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
21
]
],
"date-time": "2023-12-21T00:00:00Z",
"timestamp": 1703116800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2673-4591/55/1/81/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
12,
21
]
]
},
"published-online": {
"date-parts": [
[
2023,
12,
21
]
]
},
"publisher": "MDPI",
"publisher-location": "Basel Switzerland",
"reference": [
{
"DOI": "10.1021/acs.jnatprod.0c01324",
"article-title": "Anti-SARS-CoV-2 activity of andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives",
"author": "Suksatu",
"doi-asserted-by": "crossref",
"first-page": "1261",
"journal-title": "J. Nat. Prod.",
"key": "ref_1",
"volume": "84",
"year": "2021"
},
{
"DOI": "10.1080/J157v03n01_04",
"article-title": "A randomized, controlled study of Kan Jang versus amantadine in the treatment of influenza in Volgograd",
"author": "Kulichenko",
"doi-asserted-by": "crossref",
"first-page": "77",
"journal-title": "J. Herb. Pharmacother.",
"key": "ref_2",
"volume": "3",
"year": "2003"
},
{
"DOI": "10.1016/j.phymed.2009.12.001",
"article-title": "A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmCold) in patients with uncomplicated upper respiratory tract infection",
"author": "Saxena",
"doi-asserted-by": "crossref",
"first-page": "178",
"journal-title": "Phytomedicine",
"key": "ref_3",
"volume": "17",
"year": "2010"
},
{
"article-title": "Efficacy of Andrographis paniculata, Nees for pharyngotonsillistis in adults",
"author": "Thamlikitkul",
"first-page": "437",
"journal-title": "J. Med. Assoc.",
"key": "ref_4",
"volume": "74",
"year": "1991"
},
{
"DOI": "10.26502/aimr.0125",
"article-title": "Efficacy and safety of Andrographis paniculata extract in patients with mild COVID-19: A randomized controlled trial",
"author": "Wanaratna",
"doi-asserted-by": "crossref",
"first-page": "423",
"journal-title": "Arch. Intern. Med. Res.",
"key": "ref_5",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1002/psp4.12543",
"article-title": "Timing of antiviral treatment initiation is critical to reduce SARS-CoV-2 viral load",
"author": "Bertrand",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "CPT Pharmacomet. Syst. Pharmacol.",
"key": "ref_6",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1111/bcp.14486",
"article-title": "Model-informed drug repurposing: Viral kinetic modelling to prioritize rational drug combinations for COVID-19",
"author": "Dodds",
"doi-asserted-by": "crossref",
"first-page": "3439",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "ref_7",
"volume": "87",
"year": "2021"
},
{
"key": "ref_8",
"unstructured": "Ducharme, M.P., and Shargel, L. (2022). Shargel and Yu’s Applied Biopharmaceutics and Pharmacokinetics, McGraw Hill. [8th ed.]."
},
{
"DOI": "10.1016/S0944-7113(00)80054-9",
"article-title": "Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human",
"author": "Panossian",
"doi-asserted-by": "crossref",
"first-page": "351",
"journal-title": "Phytomedicine",
"key": "ref_9",
"volume": "7",
"year": "2000"
}
],
"reference-count": 9,
"references-count": 9,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2673-4591/55/1/81"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": "Dynamic Model of Andrographolide Therapy for COVID-19",
"type": "proceedings-article"
}