Homo-harringtonine (HHT) is highly effective against SARS-CoV-2-A potential first-line defense in future coronavirus epidemics
et al., National Science Review, doi:10.1093/nsr/nwae382, ChiCTR2100049182, Oct 2024
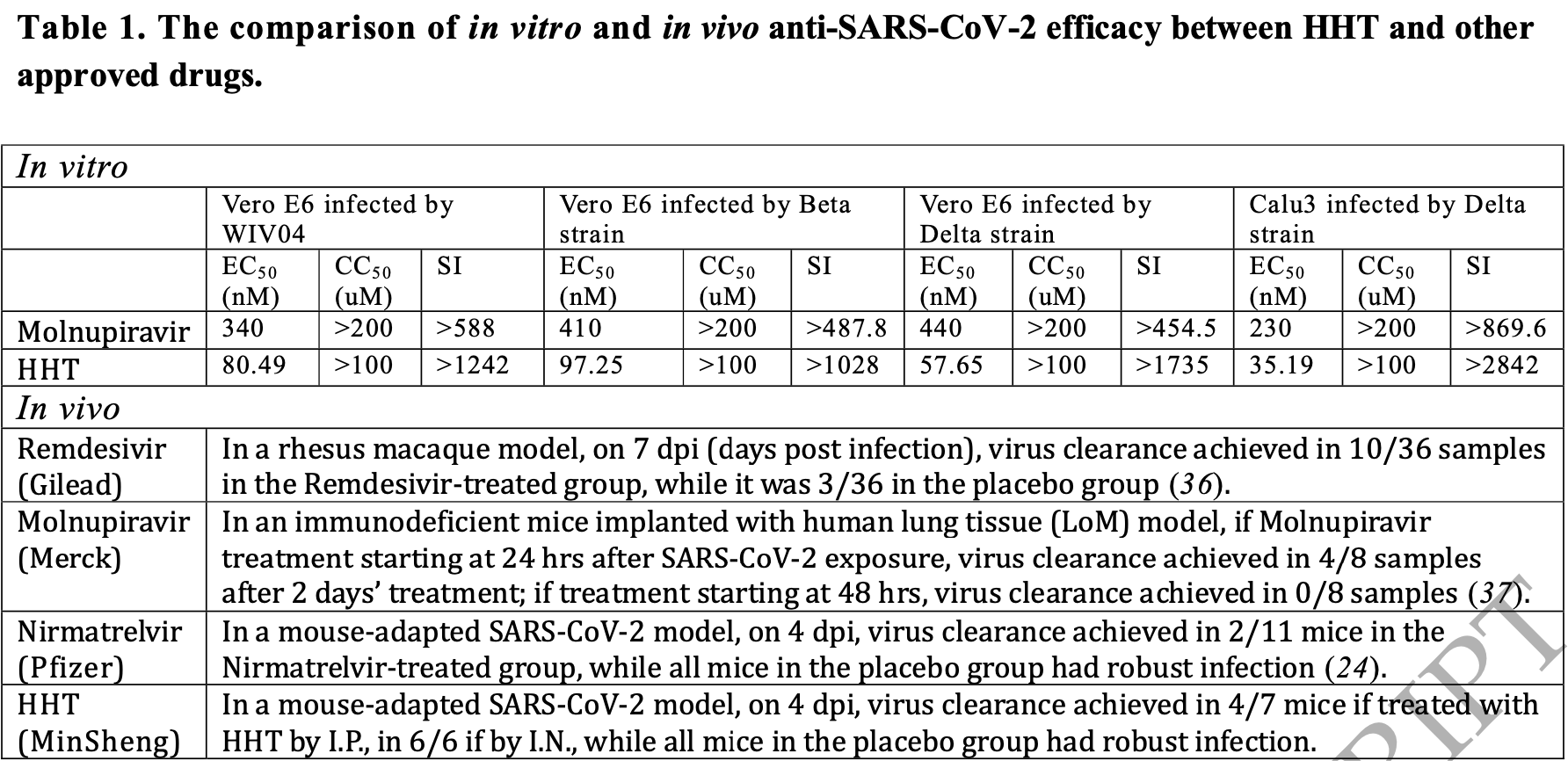
In vitro, mouse, and small clinical study showing efficacy of homoharringtonine (HHT) against SARS-CoV-2 and other coronaviruses by inhibiting protein elongation. In vitro, HHT was effective against multiple SARS-CoV-2 variants and other coronaviruses at nanomolar concentrations. In mice, intranasal or intraperitoneal HHT cleared the virus in 3-5 days. In a clinical trial of 11 newly infected patients without other conditions, 10 cleared the virus in 2-4 days with low-dose intranasal HHT, compared to 7-9 days in other studies. No adverse effects were observed. Authors propose HHT as a potential first-line defense in future coronavirus epidemics.
Wen et al., 26 Oct 2024, China, peer-reviewed, 34 authors, trial ChiCTR2100049182.
Contact: wzhongyi@mail.sysu.edu.cn, purecai@163.com, wangml@wh.iov.cn, lij@mspharm.com.
Homo-harringtonine (HHT) is highly effective against SARS-CoV-2 -A potential first-line defense in future coronavirus epidemics
doi:10.1093/nsr/nwae382/7845890
As COVID-19 is the third coronavirus epidemic in this century, it would be desirable to have a treatment scheme in anticipation of a fourth one. We here present a scheme that could clear SARS-CoV-2 in 2-4 days post infection from the upper respiratory tract (URT), where the virions are initially concentrated. The scheme, applicable in large scale by nasal spray, is based on Homoharringtonine (HHT) that has been approved for treating other diseases. HHT blocked protein elongation and repressed in vitro replication of all 4 coronaviruses (including SARS-CoV-2) tested at the nano-molar concentration, demonstrating its potential of broad effectiveness against coronaviruses. In animal models, HHT cleared SARS-CoV-2 in all treated mice in 3 days by daily nasal dripping of a small dose (40 ug). In December 2022, HHT was administered to 26 cancer patients by nebulization at 1 mg/day. On average, the viral load in the URT was reduced by 3/4 six hours after the nebulization. In the wavelet of May 2023, 11 patients without other medical conditions were administered HHT by repeated liquid nasal spray at the low, total daily-dose of 0.2 mg. Ten of the 11 patients were cleared of the virus in 2-4 days. In comparison, in large-cohort studies of participants in China during the same wave, most patients need 7-9 to turn negative. No adverse effects were detected in any patient in the two clinical trials. A short review of drugs approved for treating COVID-19 shows the many advantages of HHT. With continual development, it could become a first-line defense at the onset of future coronavirus epidemics.
Competing interests: Jian Li is an employee of MinSheng Pharmaceuticals Co., Ltd. The other authors declare that they have no competing interests.
References
Abdelnabi, The combined treatment of Molnupiravir and Favipiravir in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model, EBioMedicine
Al-Tawfiq, Asymptomatic coronavirus infection: MERS-CoV and SARS-CoV-2 (COVID-19), Travel medicine and infectious disease
Alvandi, Food and Drug Administration approval summary: omacetaxine mepesuccinate as treatment for chronic myeloid leukemia, Oncologist
Andersen, Novel Antiviral Activities of Obatoclax, Emetine, Niclosamide, Brequinar, and Homoharringtonine, Viruses
Bhatt, Structural basis of ribosomal frameshifting during translation of the SARS-CoV-2 RNA genome, Science
Brocchieri, Karlin, Protein length in eukaryotic and prokaryotic proteomes, Nucleic Acids Res
Butler, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, The Lancet
Cao, Forrest, Zhang, A screen of the NIH Clinical Collection small molecule library identifies potential anti-coronavirus drugs, Antiviral Res
Cao, Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Nature
Cao, Oral Simnotrelvir for Adult Patients with Mild-to-Moderate Covid-19, New England Journal of Medicine
Cao, Was Wuhan the early epicenter of the COVID-19 pandemic?-A critique, National Science Review
Consortium, Repurposed Antiviral Drugs for Covid-19 -Interim WHO Solidarity Trial Results, N Engl J Med
Dong, The Natural Compound Homoharringtonine Presents Broad Antiviral Activity In Vitro and In Vivo, Viruses
Dyall, Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection, Antimicrob Agents Chemother
Fan, Oral VV116 versus placebo in patients with mild-to-moderate COVID-19 in China: a multicentre, double-blind, phase 3, randomised controlled study, The Lancet Infectious Diseases
Fresno, Jimenez, Vazquez, Inhibition of translation in eukaryotic systems by harringtonine, Eur J Biochem
Gandhi, Plunkett, Cortes, Omacetaxine: a protein translation inhibitor for treatment of chronic myelogenous leukemia, Clin Cancer Res
Garreau De Loubresse, Structural basis for the inhibition of the eukaryotic ribosome, Nature
Goldman, Gonzalez, Ruthrich, Sharon, Von Lilienfeld-Toal, COVID-19 and Cancer: Special Considerations for Patients Receiving Immunotherapy and Immunosuppressive Cancer Therapies, Am Soc Clin Oncol Educ Book
Hammond, Nirmatrelvir for vaccinated or unvaccinated adult outpatients with Covid-19, New England Journal of Medicine
Hammond, Oral Nirmatrelvir-Ritonavir as Postexposure Prophylaxis for Covid-19, New England Journal of Medicine
He, Temporal dynamics in viral shedding and transmissibility of COVID-19, Nature medicine
Hou, Intra-vs. Interhost of SARS-CoV-2 Driven by Uncorrelated Selection-The Evolution Thwarted, Molecular Biology and Evolution
Ianevski, Potential Antiviral Options against SARS-CoV-2 Infection, Viruses
Li, Hilgenfeld, Whitley, Clercq, Therapeutic strategies for COVID-19: progress and lessons learned, Nature Reviews Drug Discovery
Ma, Homo-harringtonine, highly effective against coronaviruses, is safe in treating COVID-19 by nebulization, Sci China Life Sci
Meister, Virucidal Efficacy of Different Oral Rinses Against Severe Acute Respiratory Syndrome Coronavirus 2, J Infect Dis
Owen, An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19, Science
Perlman, Mcintosh, Coronaviruses, including severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS)
Planas, Distinct evolution of SARS-CoV-2 Omicron XBB and BA. 2.86/JN. 1 lineages combining increased fitness and antibody evasion, Nature Communications
Qiao, SARS-CoV-2 Mpro inhibitors with antiviral activity in a transgenic mouse model, Science
Research, Cephalotaxine esters in the treatment of acute leukemia. A preliminary clinical assessment, Chin Med J
Ruan, On the epicenter of COVID-19 and the origin of the pandemic strain, National Science Review
Ruan, The twin-beginnings of COVID-19 in Asia and Europe-One prevails quickly, Natl Sci Rev
Shuai, Replication, pathogenicity, and transmission of SARS-CoV-2 in minks, Natl Sci Rev
Vangeel, Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern, Antiviral Res
Velkov, Abdul Rahim, Zhou, Chan, Li, Inhaled anti-infective chemotherapy for respiratory tract infections: successes, challenges and the road ahead, Adv Drug Deliv Rev
W.-J. Guan, Zhong, None
Wahl, SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801, Nature
Wang, A deep proteome and transcriptome abundance atlas of 29 healthy human tissues, Mol Syst Biol
Wang, Mouse-adapted SARS-CoV-2 replicates efficiently in the upper and lower respiratory tract of BALB/c and C57BL/6J mice, Protein Cell
Wang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wen, A proposal for clinical trials of COVID-19 treatment using homo-harringtonine, Natl Sci Rev
White, Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A, Science
Williamson, Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2, Nature
Wolfel, Virological assessment of hospitalized patients with COVID-2019, Nature
Woo, Huang, Lau, Yuen, Coronavirus genomics and bioinformatics analysis, Viruses
Wu, Wen, Heightened protein-translation activities in mammalian cells and the disease/treatment implications, Natl Sci Rev
Xu, Aloe extract inhibits porcine epidemic diarrhea virus in vitro and in vivo, Vet Microbiol
Zeng, Metabolomic analysis of porcine intestinal epithelial cells during swine acute diarrhea syndrome coronavirus infection, Front Cell Infect Microbiol
Zhao, A cell-based assay to discover inhibitors of SARS-CoV-2 RNA dependent RNA polymerase, Antiviral Res
Zhou, A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature
Zhou, A third dose of inactivated SARS-CoV-2 vaccine induces robust antibody responses in people with inadequate response to two-dose vaccination, National science review
DOI record:
{
"DOI": "10.1093/nsr/nwae382",
"ISSN": [
"2095-5138",
"2053-714X"
],
"URL": "http://dx.doi.org/10.1093/nsr/nwae382",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>As COVID-19 is the third coronavirus epidemic in this century, it would be desirable to have a treatment scheme in anticipation of a fourth one. We here present a scheme that could clear SARS-CoV-2 in 2-4 days post infection from the upper respiratory tract (URT), where the virions are initially concentrated. The scheme, applicable in large scale by nasal spray, is based on Homo-harringtonine (HHT) that has been approved for treating other diseases. HHT blocked protein elongation and repressed in vitro replication of all 4 coronaviruses (including SARS-CoV-2) tested at the nano-molar concentration, demonstrating its potential of broad effectiveness against coronaviruses. In animal models, HHT cleared SARS-CoV-2 in all treated mice in 3 days by daily nasal dripping of a small dose (40 ug). In December 2022, HHT was administered to 26 cancer patients by nebulization at 1 mg/day. On average, the viral load in the URT was reduced by 3/4 six hours after the nebulization. In the wavelet of May 2023, 11 patients without other medical conditions were administered HHT by repeated liquid nasal spray at the low, total daily-dose of 0.2 mg. Ten of the 11 patients were cleared of the virus in 2-4 days. In comparison, in large-cohort studies of participants in China during the same wave, most patients need 7-9 to turn negative. No adverse effects were detected in any patient in the two clinical trials. A short review of drugs approved for treating COVID-19 shows the many advantages of HHT. With continual development, it could become a first-line defense at the onset of future coronavirus epidemics.</jats:p>",
"author": [
{
"affiliation": [
{
"name": "State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University , Guangzhou 510275 ,",
"place": [
"China"
]
}
],
"family": "Wen",
"given": "Hai-Jun",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Cancer Center, Clifford Hospital, Jinan University , Guangzhou ,",
"place": [
"People's Republic of China"
]
}
],
"family": "Ma",
"given": "Hua-Juan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0748-9641",
"affiliation": [
{
"name": "State Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Researched Institute, Chinese Academy of Agricultural Sciences , Harbin 150069 ,",
"place": [
"People's Republic of China"
]
}
],
"authenticated-orcid": false,
"family": "Zhong",
"given": "Gong-Xun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University , Guangzhou 510275 ,",
"place": [
"China"
]
}
],
"family": "Ding",
"given": "Ao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Virology, Wuhan Institute of Virology, Chinese Academy of Sciences , Wuhan, 430071 ,",
"place": [
"China"
]
}
],
"family": "Wang",
"given": "Xi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University , Guangzhou 510275 ,",
"place": [
"China"
]
}
],
"family": "Lai",
"given": "Xiao-Ju",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University , Guangzhou 510275 ,",
"place": [
"China"
]
}
],
"family": "Shi",
"given": "Chang-Hao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University , Guangzhou 510275 ,",
"place": [
"China"
]
}
],
"family": "Tracy",
"given": "Miles",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Academy of Pharmacy, Xi'an Jiaotong-Liverpool University , Suzhou 215123 ,",
"place": [
"China"
]
}
],
"family": "Wu",
"given": "Si-Jin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University , Guangzhou 510275 ,",
"place": [
"China"
]
}
],
"family": "Li",
"given": "Jia-Sheng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cancer Center, Clifford Hospital, Jinan University , Guangzhou ,",
"place": [
"People's Republic of China"
]
}
],
"family": "Zhang",
"given": "Ge",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cancer Center, Clifford Hospital, Jinan University , Guangzhou ,",
"place": [
"People's Republic of China"
]
}
],
"family": "Bo Yao",
"given": "Yong-",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cancer Center, Clifford Hospital, Jinan University , Guangzhou ,",
"place": [
"People's Republic of China"
]
}
],
"family": "Chen",
"given": "Wei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University , Guangzhou 510275 ,",
"place": [
"China"
]
}
],
"family": "Yu Liu",
"given": "Xue-",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University , Guangzhou 510275 ,",
"place": [
"China"
]
}
],
"family": "Xu",
"given": "Zhi-Chao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Laboratory Animal Science, Kunming Medical University , Kunming 650500 ,",
"place": [
"People's Republic of China"
]
}
],
"family": "Cao",
"given": "Xue",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Science , Kunming 650223 ,",
"place": [
"China"
]
}
],
"family": "He",
"given": "Wen-Bin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Science , Kunming 650223 ,",
"place": [
"China"
]
}
],
"family": "Feng",
"given": "Jing",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9407-4363",
"affiliation": [
{
"name": "State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Science , Kunming 650223 ,",
"place": [
"China"
]
}
],
"authenticated-orcid": false,
"family": "Wang",
"given": "Guo-Dong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Key Laboratory of Animal Models and Human Disease Mechanisms of Chinese Academy of Sciences / Key Laboratory of Bioactive Peptides of Yunnan Province, Kunming Institute of Zoology, Chinese Academy of Sciences , Kunming, Yunnan 650223 ,",
"place": [
"China"
]
},
{
"name": "Kunming National High-level Bio-safety Research Center for Non-human Primates, Center for Biosafety Mega-Science, Kunming Institute of Zoology, Chinese Academy of Sciences , Kunming, 650107 ,",
"place": [
"China"
]
}
],
"family": "Liu",
"given": "Feng-Liang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University , Guangzhou 510275 ,",
"place": [
"China"
]
}
],
"family": "Zeng",
"given": "Wei-Shun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University , Guangzhou 510275 ,",
"place": [
"China"
]
}
],
"family": "Lu",
"given": "Guang-An",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University , Guangzhou 510275 ,",
"place": [
"China"
]
}
],
"family": "Yu Guo",
"given": "Jia-",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0345-1945",
"affiliation": [
{
"name": "Key Laboratory of Animal Models and Human Disease Mechanisms of Chinese Academy of Sciences / Key Laboratory of Bioactive Peptides of Yunnan Province, Kunming Institute of Zoology, Chinese Academy of Sciences , Kunming, Yunnan 650223 ,",
"place": [
"China"
]
},
{
"name": "Kunming National High-level Bio-safety Research Center for Non-human Primates, Center for Biosafety Mega-Science, Kunming Institute of Zoology, Chinese Academy of Sciences , Kunming, 650107 ,",
"place": [
"China"
]
}
],
"authenticated-orcid": false,
"family": "Zheng",
"given": "Yong-Tang",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7456-6061",
"affiliation": [
{
"name": "Beijing Ditan Hospital, Capital Medical University , Beijing 100102 ,",
"place": [
"China"
]
}
],
"authenticated-orcid": false,
"family": "Zeng",
"given": "Hui",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1050-802X",
"affiliation": [
{
"name": "State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University , Guangzhou 510275 ,",
"place": [
"China"
]
}
],
"authenticated-orcid": false,
"family": "He",
"given": "Xiong-Lei",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6386-9879",
"affiliation": [
{
"name": "Beijing Ditan Hospital, Capital Medical University , Beijing 100102 ,",
"place": [
"China"
]
}
],
"authenticated-orcid": false,
"family": "Zhang",
"given": "Fu-Jie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Virology, Wuhan Institute of Virology, Chinese Academy of Sciences , Wuhan, 430071 ,",
"place": [
"China"
]
}
],
"family": "Hu",
"given": "Zhi-Hong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Science , Kunming 650223 ,",
"place": [
"China"
]
}
],
"family": "Lyu",
"given": "Xue-Mei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Researched Institute, Chinese Academy of Agricultural Sciences , Harbin 150069 ,",
"place": [
"People's Republic of China"
]
}
],
"family": "Chen",
"given": "Hualan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hangzhou Minsheng Pharm. Group , Hangzhou ,",
"place": [
"China"
]
}
],
"family": "Li",
"given": "Jian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Virology, Wuhan Institute of Virology, Chinese Academy of Sciences , Wuhan, 430071 ,",
"place": [
"China"
]
}
],
"family": "Wang",
"given": "Man-Li",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cancer Center, Clifford Hospital, Jinan University , Guangzhou ,",
"place": [
"People's Republic of China"
]
}
],
"family": "Cai",
"given": "Qi-Chun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University , Guangzhou 510275 ,",
"place": [
"China"
]
},
{
"name": "Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai) , Zhuhai 519082 ,",
"place": [
"People's Republic of China"
]
}
],
"family": "Wu",
"given": "Chung-I",
"sequence": "additional"
}
],
"container-title": "National Science Review",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
10,
27
]
],
"date-time": "2024-10-27T04:35:38Z",
"timestamp": 1730003738000
},
"deposited": {
"date-parts": [
[
2024,
10,
27
]
],
"date-time": "2024-10-27T04:35:38Z",
"timestamp": 1730003738000
},
"indexed": {
"date-parts": [
[
2024,
10,
27
]
],
"date-time": "2024-10-27T05:10:10Z",
"timestamp": 1730005810469,
"version": "3.28.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
10,
26
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "am",
"delay-in-days": 1,
"start": {
"date-parts": [
[
2024,
10,
27
]
],
"date-time": "2024-10-27T00:00:00Z",
"timestamp": 1729987200000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/nsr/advance-article-pdf/doi/10.1093/nsr/nwae382/60135373/nwae382.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/nsr/advance-article-pdf/doi/10.1093/nsr/nwae382/60135373/nwae382.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2024,
10,
26
]
]
},
"published-online": {
"date-parts": [
[
2024,
10,
26
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/nsr/advance-article/doi/10.1093/nsr/nwae382/7845890"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Homo-harringtonine (HHT) is highly effective against SARS-CoV-2-A potential first-line defense in future coronavirus epidemics",
"type": "journal-article"
}