A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward
et al., Journal of Hematology & Oncology, doi:10.1186/s13045-020-00934-x, Jul 2020
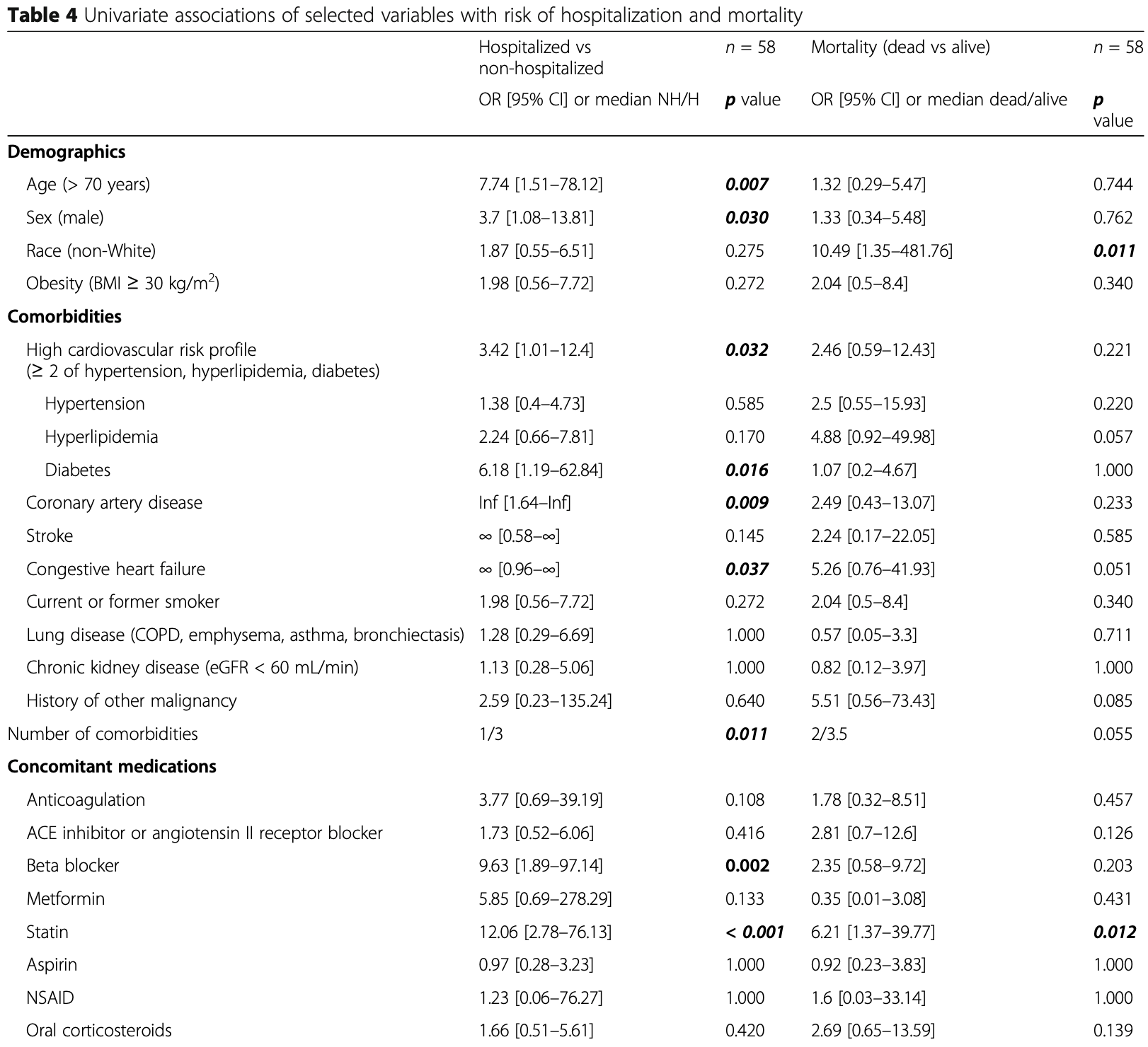
Retrospective 58 multiple myeloma COVID-19 patients in the USA, showing no significant difference with aspirin treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
Study covers aspirin and metformin.
|
risk of death, 57.7% lower, RR 0.42, p = 0.43, treatment 1 of 9 (11.1%), control 13 of 49 (26.5%), NNT 6.5, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Wang et al., 14 Jul 2020, retrospective, USA, peer-reviewed, 13 authors.
A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward
Journal of Hematology & Oncology, doi:10.1186/s13045-020-00934-x
Background: The COVID-19 pandemic, caused by SARS-CoV-2 virus, has resulted in over 100,000 deaths in the USA. Our institution has treated over 2000 COVID-19 patients during the pandemic in New York City. The pandemic directly impacted cancer patients and the organization of cancer care. Mount Sinai Hospital has a large and diverse multiple myeloma (MM) population. Herein, we report the characteristics of COVID-19 infection and serological response in MM patients in a large tertiary care institution in New York. Methods: We performed a retrospective study on a cohort of 58 patients with a plasma-cell disorder (54 MM, 4 smoldering MM) who developed COVID-19 between March 1, 2020, and April 30, 2020. We report epidemiological, clinical, and laboratory characteristics including the persistence of viral detection by polymerase chain reaction (PCR) and anti-SARS-CoV-2 antibody testing, treatments initiated, and outcomes. Results: Of the 58 patients diagnosed with COVID-19, 36 were hospitalized and 22 were managed at home. The median age was 67 years; 52% of patients were male and 63% were non-White. Hypertension (64%), hyperlipidemia (62%), obesity (37%), diabetes mellitus (28%), chronic kidney disease (24%), and lung disease (21%) were the most common comorbidities. In the total cohort, 14 patients (24%) died. Older age (> 70 years), male sex, cardiovascular risk, and patients not in complete remission (CR) or stringent CR were significantly (p < 0.05) associated with hospitalization. Among hospitalized patients, laboratory findings demonstrated elevation of traditional inflammatory markers (CRP, ferritin, D-dimer) and a significant (p < 0.05) association between elevated inflammatory markers, severe hypogammaglobulinemia, non-White race, and mortality. Ninety-six percent (22/23) of patients developed antibodies to SARS-CoV-2 at a median of 32 days after initial diagnosis. The median time to PCR negativity was 43 (range 19-68) days from initial positive PCR.
Supplementary information Supplementary information accompanies this paper at https://doi.org/10. 1186/s13045-020-00934-x. Additional file 1: Figure S1 . Evolution of selected inflammatory biomarkers in a subset of patients (n = 12) hospitalized at the Mount Sinai Hospital for which the data was available. Different measurements from the same patient are connected. A linear regression line is plotted for the subgroup of patients that survived (blue, n = 8) and died (red, n = 4), respectively.
Abbreviations
Funding There is no outside funding declared for this study.
Ethics approval and consent to participate This study was performed in accordance with the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice (IRB: GCO#: 11-1433).
Consent for publication Not applicable. Author details
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Bryce, Grimes, Pujadas, Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response, medRxiv
Cao, Wei, Zou, Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial, J Allergy Clin Immunol
Cook, Ashcroft, Pratt, Real-world assessment of the clinical impact of symptomatic infection with severe acute respiratory syndrome coronavirus (COVID-19 disease) in patients with Multiple Myeloma receiving systemic anti-cancer therapy, Br J Haematol
Costa, Ws, Bermúdez, First clinical study of the B-cell maturation antigen (BCMA) 2 + 1 T cell engager (TCE) CC-93269 in patients (pts) with relapsed/refractory multiple myeloma (RRMM): interim results of a phase 1 multicenter trial. Abstract #143
Dai, Liu, Liu, Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak, Cancer Discov
Dai, Wu, Jia, Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia, J Hematol Oncol
Gong, Dong, Xia, Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia, medRxiv
Gross, Essien, Pasha, Gross, Wang et al., Racial and ethnic disparities in population level COVID-19 mortality, medRxiv
Henning-Smith, Tuttle, Kozhimannil, Unequal distribution of COVID-19 risk among rural residents by race and ethnicity, J Rural Health
Howard, Safford, Moy, Racial differences in the incidence of cardiovascular risk factors in older Black and White adults, J Am Geriatr Soc
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Ingraham, Lotfi-Emran, Thielen, Immunomodulation in COVID-19, Lancet Respir Med
Jatiani, Aleman, Madduri, Myeloma CAR-T CRS management with IL-1R antagonist anakinra, Clin Lymphoma MyelomaLeukemia
Kumar, Anderson, Immune therapies in multiple myeloma, Clin Cancer Res
Kumar, Paiva, Anderson, International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma, Lancet Oncol
Kyle, Rajkumar, Multiple myeloma, Blood
Lee, Borrello, Role of the immune response in disease progression and therapy in multiple myeloma, Cancer Treat Res
Lee, Santomasso, Locke, ASTCT Consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells, Biol Blood Marrow Transplant
Liang, Guan, Chen, Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China, Lancet Oncol
Liu, Zhao, Cytokine release syndrome: grading, modeling, and new therapy, J Hematol Oncol
Malard, Mohty, Management of patients with multiple myeloma during the COVID-19 pandemic, Lancet Haematol
Marie, Valle, Kim-Schulze, Huang, An inflammatory cytokine signature helps predict COVID-19 severity and death, MEDRXIV
Mehta, Mcauley, Brown, COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet
Merad, Martin, Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages, Nat Rev Immunol
Rajkumar, Dimopoulos, Palumbo, International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma, Lancet Oncol
Richardson, Hirsch, Narasimhan, Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area, JAMA
Robilotti, Babady, Mead, Determinants of severity in cancer patients with COVID-19 illness, medRxiv
Saleh, Sher, Gertz, Multiple myeloma in the time of COVID-19, Acta Haematol
Society, Recommendations for the management of myeloma patients during the COVID-19 pandemic
Sørrig, Klausen, Salomo, Smoldering multiple myeloma risk factors for progression: a Danish population-based cohort study, Eur J Haematol
Tseng, Perrone, Zhu, Makino, Peters, Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection, J Immunol
Widman, Gornisiewicz, Shacham, Tamir, In vitro toxicity and efficacy of verdinexor, an exportin 1 inhibitor, on opportunistic viruses affecting immunocompromised individuals, PLoS One
Wu, Gui, Feng, KPT-330, a potent and selective CRM1 inhibitor, exhibits anti-inflammation effects and protection against sepsis, Biochem Biophys Res Commun
Xu, Han, Li, Effective treatment of severe COVID-19 patients with tocilizumab, Proc Natl Acad Sci
Yu, Ouyang, Chua, Xie, SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China, JAMA Oncol
Zhang, Wu, Li, Zhao, Wang, The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality, Int J Antimicrob Agents
Zhang, Zhu, Xie, Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China, Ann Oncol
DOI record:
{
"DOI": "10.1186/s13045-020-00934-x",
"ISSN": [
"1756-8722"
],
"URL": "http://dx.doi.org/10.1186/s13045-020-00934-x",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n<jats:title>Background</jats:title>\n<jats:p>The COVID-19 pandemic, caused by SARS-CoV-2 virus, has resulted in over 100,000 deaths in the USA. Our institution has treated over 2000 COVID-19 patients during the pandemic in New York City. The pandemic directly impacted cancer patients and the organization of cancer care. Mount Sinai Hospital has a large and diverse multiple myeloma (MM) population. Herein, we report the characteristics of COVID-19 infection and serological response in MM patients in a large tertiary care institution in New York.</jats:p>\n</jats:sec><jats:sec>\n<jats:title>Methods</jats:title>\n<jats:p>We performed a retrospective study on a cohort of 58 patients with a plasma-cell disorder (54 MM, 4 smoldering MM) who developed COVID-19 between March 1, 2020, and April 30, 2020. We report epidemiological, clinical, and laboratory characteristics including the persistence of viral detection by polymerase chain reaction (PCR) and anti-SARS-CoV-2 antibody testing, treatments initiated, and outcomes.</jats:p>\n</jats:sec><jats:sec>\n<jats:title>Results</jats:title>\n<jats:p>Of the 58 patients diagnosed with COVID-19, 36 were hospitalized and 22 were managed at home. The median age was 67 years; 52% of patients were male and 63% were non-White. Hypertension (64%), hyperlipidemia (62%), obesity (37%), diabetes mellitus (28%), chronic kidney disease (24%), and lung disease (21%) were the most common comorbidities. In the total cohort, 14 patients (24%) died. Older age (> 70 years), male sex, cardiovascular risk, and patients not in complete remission (CR) or stringent CR were significantly (<jats:italic>p</jats:italic> < 0.05) associated with hospitalization. Among hospitalized patients, laboratory findings demonstrated elevation of traditional inflammatory markers (CRP, ferritin, D-dimer) and a significant (<jats:italic>p</jats:italic> < 0.05) association between elevated inflammatory markers, severe hypogammaglobulinemia, non-White race, and mortality. Ninety-six percent (22/23) of patients developed antibodies to SARS-CoV-2 at a median of 32 days after initial diagnosis. The median time to PCR negativity was 43 (range 19–68) days from initial positive PCR.</jats:p>\n</jats:sec><jats:sec>\n<jats:title>Conclusions</jats:title>\n<jats:p>Drug exposure and MM disease status at the time of contracting COVID-19 had no bearing on mortality. Mounting a severe inflammatory response to SARS-CoV-2 and severe hypogammaglobulinemia was associated with higher mortality. The majority of patients mounted an antibody response to SARS-CoV-2. These findings pave a path to the identification of vulnerable MM patients who need early intervention to improve outcomes in future outbreaks of COVID-19.</jats:p>\n</jats:sec>",
"alternative-id": [
"934"
],
"article-number": "94",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "31 May 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "3 July 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "14 July 2020"
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "This study was performed in accordance with the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice (IRB: GCO#: 11-1433)."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "A.C.: Advisory board and consulting fees from Amgen, Antegene, Celgene, Janssen, Karyopharm, Millennium/Takeda, Novartis Pharmaceuticals, Oncopeptides, Sanofi; research funding from Amgen, Celgene, Janssen, Millennium/Takeda, Novartis Pharmaceuticals, Pharmacyclics. S. J.: Advisory board and consulting fees from Celgene, Bristol-Myers Squibb, Janssen Pharmaceuticals and Merck. H. J. C: Employed by the Multiple Myeloma Research Foundation, advisory board and consulting fees from Genetech, Celgene, Bristol Myers Squibb, GlaxoSmithKline and received research funding from Takeda, Celgene, and Genetech. D. M.: Advisory board and consulting fees from Janssen, Celgene, Bristol Myers Squibb, Takeda, Legend, GlaxoSmithKline, Kinevant, and Foundation Medicine. B.W.: Consulting fees from Sanofi Genzyme. J. R.: Speaking fees from Celgene and Janssen, advisory board and consulting fees from Celgene, Janssen, Bristol Myers Squibb, Oncopeptides, Adaptive Biotechnologies, X4 Pharmaceuticals, Karyopharm, and Antegene. S. P.: Consulting fees from Foundation Medicine, research funding from Celgene and Karyopharm, supported by 1R01CA244899-01A1. All other authors declare no potential conflict of interest<b>.</b>"
}
],
"author": [
{
"affiliation": [],
"family": "Wang",
"given": "Bo",
"sequence": "first"
},
{
"affiliation": [],
"family": "Van Oekelen",
"given": "Oliver",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mouhieddine",
"given": "Tarek H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Del Valle",
"given": "Diane Marie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richter",
"given": "Joshua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cho",
"given": "Hearn Jay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richard",
"given": "Shambavi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chari",
"given": "Ajai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gnjatic",
"given": "Sacha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Merad",
"given": "Miriam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jagannath",
"given": "Sundar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parekh",
"given": "Samir",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7305-9690",
"affiliation": [],
"authenticated-orcid": false,
"family": "Madduri",
"given": "Deepu",
"sequence": "additional"
}
],
"container-title": "Journal of Hematology & Oncology",
"container-title-short": "J Hematol Oncol",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2020,
7,
14
]
],
"date-time": "2020-07-14T13:38:34Z",
"timestamp": 1594733914000
},
"deposited": {
"date-parts": [
[
2021,
7,
14
]
],
"date-time": "2021-07-14T00:18:56Z",
"timestamp": 1626221936000
},
"indexed": {
"date-parts": [
[
2024,
4,
2
]
],
"date-time": "2024-04-02T06:38:28Z",
"timestamp": 1712039908806
},
"is-referenced-by-count": 94,
"issue": "1",
"issued": {
"date-parts": [
[
2020,
7,
14
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2020,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
7,
14
]
],
"date-time": "2020-07-14T00:00:00Z",
"timestamp": 1594684800000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
7,
14
]
],
"date-time": "2020-07-14T00:00:00Z",
"timestamp": 1594684800000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13045-020-00934-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s13045-020-00934-x/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13045-020-00934-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2020,
7,
14
]
]
},
"published-online": {
"date-parts": [
[
2020,
7,
14
]
]
},
"published-print": {
"date-parts": [
[
2020,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "934_CR1",
"unstructured": "Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020."
},
{
"key": "934_CR2",
"unstructured": "Robilotti EV, Babady NE, Mead PA, et al. Determinants of severity in cancer patients with COVID-19 illness. medRxiv. 2020; 2020.05.04.20086322."
},
{
"DOI": "10.1158/2159-8290.CD-20-0422",
"doi-asserted-by": "crossref",
"key": "934_CR3",
"unstructured": "Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020."
},
{
"DOI": "10.1016/S1470-2045(20)30096-6",
"author": "W Liang",
"doi-asserted-by": "publisher",
"first-page": "335",
"journal-title": "Lancet Oncol",
"key": "934_CR4",
"unstructured": "Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–7.",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1101/2020.02.22.20025320",
"doi-asserted-by": "crossref",
"key": "934_CR5",
"unstructured": "Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020."
},
{
"DOI": "10.1016/j.annonc.2020.03.296",
"doi-asserted-by": "crossref",
"key": "934_CR6",
"unstructured": "Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020."
},
{
"DOI": "10.1182/blood-2007-10-078022",
"author": "RA Kyle",
"doi-asserted-by": "publisher",
"first-page": "2962",
"journal-title": "Blood",
"key": "934_CR7",
"unstructured": "Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–72.",
"volume": "111",
"year": "2008"
},
{
"DOI": "10.1007/978-3-319-40320-5_12",
"author": "SJ Lee",
"doi-asserted-by": "publisher",
"first-page": "207",
"journal-title": "Cancer Treat Res",
"key": "934_CR8",
"unstructured": "Lee SJ, Borrello I. Role of the immune response in disease progression and therapy in multiple myeloma. Cancer Treat Res. 2016;169:207–25.",
"volume": "169",
"year": "2016"
},
{
"DOI": "10.1158/1078-0432.CCR-16-0868",
"author": "SK Kumar",
"doi-asserted-by": "publisher",
"first-page": "5453",
"journal-title": "Clin Cancer Res",
"key": "934_CR9",
"unstructured": "Kumar SK, Anderson KC. Immune therapies in multiple myeloma. Clin Cancer Res. 2016;22:5453–60.",
"volume": "22",
"year": "2016"
},
{
"key": "934_CR10",
"unstructured": "Ingraham NE, Lotfi-Emran S, Thielen BK, et al. Immunomodulation in COVID-19. Lancet Respir Med."
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"doi-asserted-by": "crossref",
"key": "934_CR11",
"unstructured": "Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020."
},
{
"DOI": "10.1038/s41577-020-0331-4",
"doi-asserted-by": "crossref",
"key": "934_CR12",
"unstructured": "Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020."
},
{
"DOI": "10.1186/s13045-018-0653-x",
"author": "D Liu",
"doi-asserted-by": "publisher",
"first-page": "121",
"journal-title": "J Hematol Oncol",
"key": "934_CR13",
"unstructured": "Liu D, Zhao J. Cytokine release syndrome: grading, modeling, and new therapy. J Hematol Oncol. 2018;11:121.",
"volume": "11",
"year": "2018"
},
{
"DOI": "10.1016/j.bbmt.2018.12.758",
"author": "DW Lee",
"doi-asserted-by": "publisher",
"first-page": "625",
"journal-title": "Biol Blood Marrow Transplant",
"key": "934_CR14",
"unstructured": "Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–38.",
"volume": "25",
"year": "2019"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105954",
"doi-asserted-by": "crossref",
"key": "934_CR15",
"unstructured": "Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;105954."
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"author": "P Mehta",
"doi-asserted-by": "publisher",
"first-page": "1033",
"journal-title": "Lancet (London, England)",
"key": "934_CR16",
"unstructured": "Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England). 2020;395:1033–4.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"author": "C Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"journal-title": "Lancet (London, England)",
"key": "934_CR17",
"unstructured": "Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020;395:497–506.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1101/2020.02.25.20025643",
"doi-asserted-by": "crossref",
"key": "934_CR18",
"unstructured": "Gong J, Dong H, Xia SQ, et al. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia. medRxiv. 2020; 2020.02.25.20025643."
},
{
"DOI": "10.1016/S1470-2045(14)70442-5",
"author": "SV Rajkumar",
"doi-asserted-by": "publisher",
"first-page": "e538",
"journal-title": "Lancet Oncol",
"key": "934_CR19",
"unstructured": "Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48.",
"volume": "15",
"year": "2014"
},
{
"DOI": "10.1016/S1470-2045(16)30206-6",
"author": "S Kumar",
"doi-asserted-by": "publisher",
"first-page": "e328",
"journal-title": "Lancet Oncol",
"key": "934_CR20",
"unstructured": "Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e46.",
"volume": "17",
"year": "2016"
},
{
"DOI": "10.1111/ejh.12728",
"author": "R Sørrig",
"doi-asserted-by": "publisher",
"first-page": "303",
"journal-title": "Eur J Haematol",
"key": "934_CR21",
"unstructured": "Sørrig R, Klausen TW, Salomo M, et al. Smoldering multiple myeloma risk factors for progression: a Danish population-based cohort study. Eur J Haematol. 2016;97:303–9.",
"volume": "97",
"year": "2016"
},
{
"DOI": "10.1371/journal.pone.0200043",
"author": "DG Widman",
"doi-asserted-by": "publisher",
"first-page": "e0200043",
"journal-title": "PLoS One",
"key": "934_CR22",
"unstructured": "Widman DG, Gornisiewicz S, Shacham S, Tamir S. In vitro toxicity and efficacy of verdinexor, an exportin 1 inhibitor, on opportunistic viruses affecting immunocompromised individuals. PLoS One. 2018;13:e0200043.",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1016/j.bbrc.2018.07.112",
"author": "M Wu",
"doi-asserted-by": "publisher",
"first-page": "1773",
"journal-title": "Biochem Biophys Res Commun",
"key": "934_CR23",
"unstructured": "Wu M, Gui H, Feng Z, et al. KPT-330, a potent and selective CRM1 inhibitor, exhibits anti-inflammation effects and protection against sepsis. Biochem Biophys Res Commun. 2018;503:1773–9.",
"volume": "503",
"year": "2018"
},
{
"DOI": "10.1159/000507690",
"doi-asserted-by": "crossref",
"key": "934_CR24",
"unstructured": "Al Saleh AS, Sher T, Gertz MA. Multiple myeloma in the time of COVID-19. Acta Haematol. 2020:1–7."
},
{
"author": "IM Society",
"key": "934_CR25",
"unstructured": "Society IM. Recommendations for the management of myeloma patients during the COVID-19 pandemic; 2020.",
"volume-title": "Recommendations for the management of myeloma patients during the COVID-19 pandemic",
"year": "2020"
},
{
"DOI": "10.1016/S2352-3026(20)30124-1",
"doi-asserted-by": "crossref",
"key": "934_CR26",
"unstructured": "Malard F, Mohty M. Management of patients with multiple myeloma during the COVID-19 pandemic. Lancet Haematol. 2020."
},
{
"key": "934_CR27",
"unstructured": "COVID-19: Data. 2020. at https://www1.nyc.gov/site/doh/covid/covid-19-data.page.)."
},
{
"key": "934_CR28",
"unstructured": "Cook G, Ashcroft AJ, Pratt G, et al. Real-world assessment of the clinical impact of symptomatic infection with severe acute respiratory syndrome coronavirus (COVID-19 disease) in patients with Multiple Myeloma receiving systemic anti-cancer therapy. Br J Haematol."
},
{
"DOI": "10.1101/2020.05.07.20094250",
"doi-asserted-by": "crossref",
"key": "934_CR29",
"unstructured": "Gross CP, Essien UR, Pasha S, Gross JR, Wang S-Y, Nunez-Smith M. Racial and ethnic disparities in population level COVID-19 mortality. medRxiv. 2020; 2020.05.07.20094250."
},
{
"DOI": "10.1111/jrh.12463",
"doi-asserted-by": "crossref",
"key": "934_CR30",
"unstructured": "Henning-Smith C, Tuttle M, Kozhimannil KB. Unequal distribution of COVID-19 risk among rural residents by race and ethnicity. J Rural Health. 2020."
},
{
"DOI": "10.1111/jgs.14472",
"author": "G Howard",
"doi-asserted-by": "publisher",
"first-page": "83",
"journal-title": "J Am Geriatr Soc",
"key": "934_CR31",
"unstructured": "Howard G, Safford MM, Moy CS, et al. Racial differences in the incidence of cardiovascular risk factors in older Black and White adults. J Am Geriatr Soc. 2017;65:83–90.",
"volume": "65",
"year": "2017"
},
{
"DOI": "10.1101/2020.05.28.20115758",
"doi-asserted-by": "crossref",
"key": "934_CR32",
"unstructured": "Diane Marie Del Valle M, Seunghee Kim-Schulze PD, Hsin-Hui Huang PD, et al. An inflammatory cytokine signature helps predict COVID-19 severity and death. MEDRXIV. 2020;115758."
},
{
"DOI": "10.1016/j.clml.2020.04.020",
"doi-asserted-by": "crossref",
"key": "934_CR33",
"unstructured": "Jatiani SS, Aleman A, Madduri D, et al. Myeloma CAR-T CRS management with IL-1R antagonist anakinra. Clin Lymphoma MyelomaLeukemia. 2020."
},
{
"DOI": "10.1186/s13045-020-00856-8",
"author": "H Dai",
"doi-asserted-by": "publisher",
"first-page": "30",
"journal-title": "J Hematol Oncol",
"key": "934_CR34",
"unstructured": "Dai H, Wu Z, Jia H, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13:30.",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1182/blood-2019-122895",
"doi-asserted-by": "crossref",
"key": "934_CR35",
"unstructured": "Costa LJ WS, Bermúdez A. First clinical study of the B-cell maturation antigen (BCMA) 2 + 1 T cell engager (TCE) CC-93269 in patients (pts) with relapsed/refractory multiple myeloma (RRMM): interim results of a phase 1 multicenter trial. Abstract #143. American Society of Hematology. Orlando: ASH Annual Meeting Proceedings; 2019."
},
{
"DOI": "10.1073/pnas.2005615117",
"author": "X Xu",
"doi-asserted-by": "publisher",
"first-page": "10970",
"journal-title": "Proc Natl Acad Sci",
"key": "934_CR36",
"unstructured": "Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117:10970–5.",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1016/j.jaci.2020.05.019",
"doi-asserted-by": "crossref",
"key": "934_CR37",
"unstructured": "Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020."
},
{
"DOI": "10.4049/jimmunol.174.12.7977",
"author": "C-TK Tseng",
"doi-asserted-by": "publisher",
"first-page": "7977",
"journal-title": "J Immunol",
"key": "934_CR38",
"unstructured": "Tseng C-TK, Perrone LA, Zhu H, Makino S, Peters CJ. Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J Immunol. 2005;174:7977–85.",
"volume": "174",
"year": "2005"
},
{
"DOI": "10.1101/2020.05.18.20099960",
"doi-asserted-by": "crossref",
"key": "934_CR39",
"unstructured": "Bryce C, Grimes Z, Pujadas E, et al. Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv. 2020; 2020.05.18.20099960."
}
],
"reference-count": 39,
"references-count": 39,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2020.06.04.20122846",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://jhoonline.biomedcentral.com/articles/10.1186/s13045-020-00934-x"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Cancer Research",
"Oncology",
"Molecular Biology",
"Hematology"
],
"subtitle": [],
"title": "A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "13"
}
wang3