Phase 2 randomised placebo-controlled trial of spironolactone and dexamethasone versus dexamethasone in COVID-19 hospitalised patients in Delhi
et al., medRxiv, doi:10.1101/2022.07.01.22277163, CTRI/2021/03/031721, Jul 2022
7th treatment shown to reduce risk in
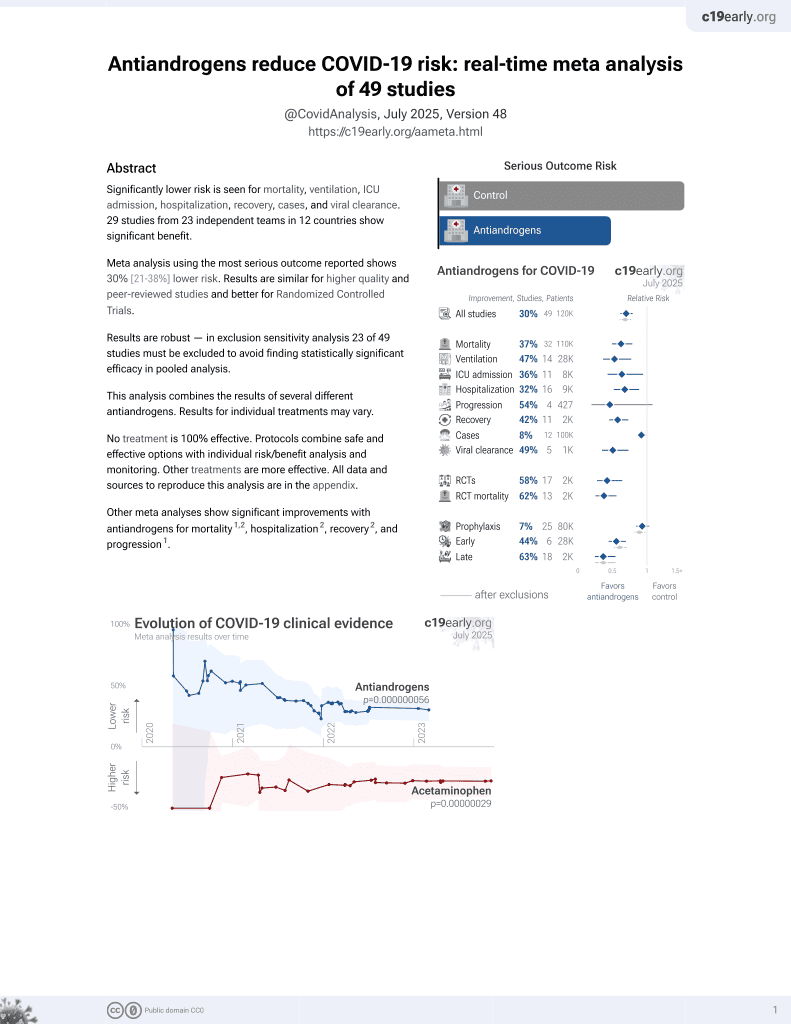
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
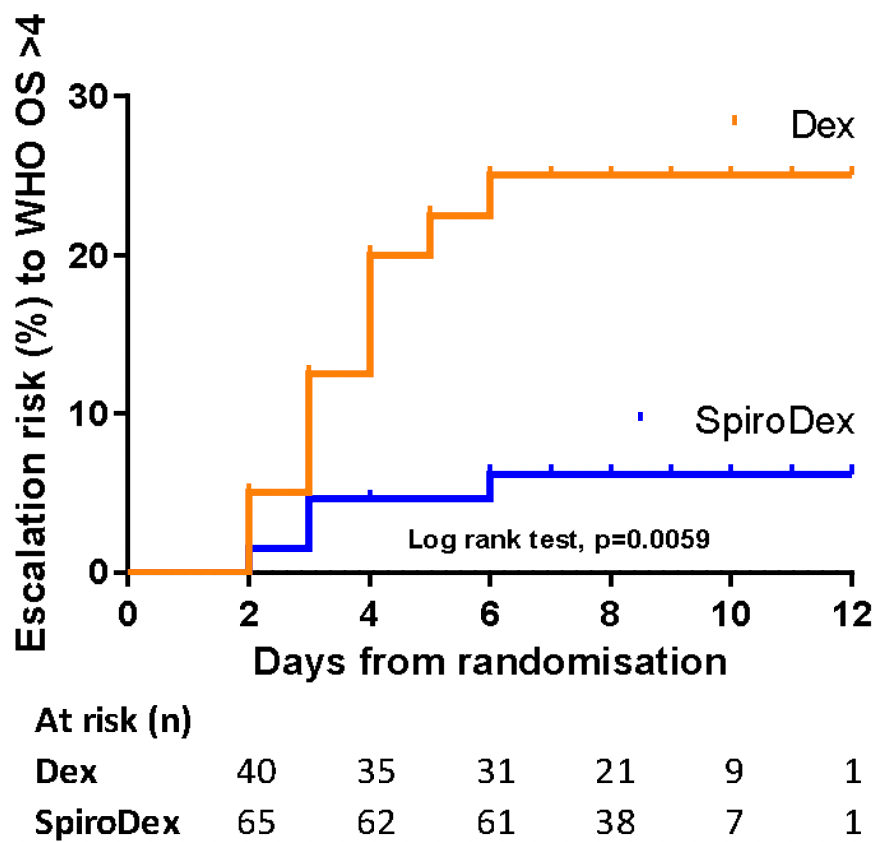
RCT 120 hospitalized patients in India, 74 treated with spironolactone and dexamethasone, and 46 with dexamethasone, showing lower progression with treatment. Spironolactone 50mg once daily day 1, 25mg once daily until day 21.
|
risk of progression, 72.4% lower, RR 0.28, p = 0.03, treatment 4 of 74 (5.4%), control 9 of 46 (19.6%), NNT 7.1, progression to WHO >4.
|
|
risk of no hospital discharge, 49.5% lower, RR 0.51, p = 0.048, treatment 13 of 74 (17.6%), control 16 of 46 (34.8%), NNT 5.8.
|
|
recovery time, 18.2% lower, relative time 0.82, p = 0.06, treatment 74, control 46.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Wadhwa et al., 2 Jul 2022, Randomized Controlled Trial, placebo-controlled, India, preprint, 18 authors, study period 1 February, 2021 - 30 April, 2021, trial CTRI/2021/03/031721.
Phase 2 randomised placebo-controlled trial of spironolactone and dexamethasone versus dexamethasone in COVID-19 hospitalised patients in Delhi
doi:10.1101/2022.07.01.22277163
Background: In this phase 2 randomised placebo-controlled clinical trial, we hypothesised that blocking mineralocorticoid receptors with spironolactone in patients with COVID-19 is safe and may reduce illness severity.
Methods: Hospitalised patients with confirmed COVID-19 were randomly allocated to low dose oral spironolactone (50mg day 1, then 25mg once daily for 21 days) or standard care in a 2:1 ratio. Both groups received dexamethasone 6mg for 10 days. Group allocation was blinded to the patient and research team. Primary outcomes were time to recovery, defined as the number of days until patients achieved WHO Ordinal Scale (OS) category ≤3, and the effect of spironolactone on aldosterone, D-dimer, angiotensin II and Von Willebrand Factor (VWF). Results: 120 patients were recruited in Delhi from 01 February to 30 April 2021. 74 were randomly assigned to spironolactone and dexamethasone (SpiroDex), and 46 to dexamethasone alone (Dex). There was no significant difference in the time to recovery between SpiroDex and Dex groups (SpiroDex median 4.5 days, Dex median 5.5 days, p = 0.055). SpiroDex patients had lower aldosterone levels on day 7 and lower D-dimer levels on days 4 and 7 (day 7 D-dimer mean SpiroDex 1.15μg/mL, Dex 3.15 μg/mL, p = 0.0004). There was no increase in adverse events in patients receiving SpiroDex. Post hoc analysis demonstrated reduced clinical deterioration (pre specified as escalating to WHO OS category >4) in the SpiroDex group vs Dex group (5.4% vs 19.6%).
Conclusion: Low dose oral spironolactone in addition to dexamethasone was safe and reduced D-Dimer and aldosterone. Although time to recovery was not significantly reduced, fewer patients progressed to severe disease. Phase 3 randomised controlled trials with spironolactone should be considered.
Author Contributions BW & VM were chief investigators of the study and responsible for all trial related activities. BW, VM, SK, FH, NP, KS, VS contributed to the implementation of the study and all study related activities in Delhi. KF, AM, JA, AB, contributed to study support and resource allocation. TQ, LF analysed the blinded data. TQ, LF, BM, MS-H, CE and KD contributed to the interpretation of the data. All authors assisted with the writing of the paper and reviewed and approved the final manuscript.
Conflict of interests The authors have no conflict of interests.
Supplemental data
Patient
References
Albitar, Ballouze, Ooi, Ghadzi, Risk factors for mortality among COVID-19 patients, Diabetes Res Clin Pract
Contou, Cally, Sarfati, Desaint, Fraissé et al., Causes and timing of death in critically ill COVID-19 patients, Critical Care
Edwards, Klekot, Halugan, Korchev, Follow Your Nose: A Key Clue to Understanding and Treating COVID-19, Front Endocrinol
Edwards, New Horizons: Does Mineralocorticoid Receptor Activation by Cortisol Cause ATP Release and COVID-19 Complications?, The Journal of Clinical Endocrinology & Metabolism
Felsenstein, Herbert, Mcnamara, Hedrich, COVID-19: Immunology and treatment options, Clin Immunol
Hansen, Rieneck, Bendtzen, Spironolactone inhibits production of proinflammatory cytokines by human mononuclear cells, Immunol Lett
Hoffmann, Schroeder, Kleine-Weber, Müller, Drosten et al., Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19, Antimicrobial Agents and Chemotherapy
Ji, Ma, Zhou, Zhang, Lu et al., Spironolactone attenuates bleomycin-induced pulmonary injury partially via modulating mononuclear phagocyte phenotype switching in circulating and alveolar compartments, PLoS One
Leach, Mohr, Giotis, Cil, Isac et al., The antiandrogen enzalutamide downregulates TMPRSS2 and reduces cellular entry of SARS-CoV-2 in human lung cells, Nature Communications
Marshall, Murthy, Diaz, Adhikari, Angus et al., A minimal common outcome measure set for COVID-19 clinical research, The Lancet Infectious Diseases
Rco, National Early Warning Score (NEWS) 2: Standardising the assessment of acute-illness severity in the NHS. Updated report of a working party
Tang, Li, Wang, Sun, Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia, J Thromb Haemost
Verdecchia, Cavallini, Spanevello, Angeli, The pivotal link between ACE2 deficiency and SARS-CoV-2 infection, Eur J Intern Med
Vicenzi, Ruscica, Iodice, Rota, Ratti et al., The Efficacy of the Mineralcorticoid Receptor Antagonist Canrenone in COVID-19 Patients, J Clin Med
Wambier, Goren, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated, J Am Acad Dermatol
Yan, Zhang, Li, Xia, Guo et al., Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2, Science
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, The Lancet
DOI record:
{
"DOI": "10.1101/2022.07.01.22277163",
"URL": "http://dx.doi.org/10.1101/2022.07.01.22277163",
"abstract": "<jats:title>ABSTRACT</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>In this phase 2 randomised placebo-controlled clinical trial, we hypothesised that blocking mineralocorticoid receptors with spironolactone in patients with COVID-19 is safe and may reduce illness severity.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Hospitalised patients with confirmed COVID-19 were randomly allocated to low dose oral spironolactone (50mg day 1, then 25mg once daily for 21 days) or standard care in a 2:1 ratio. Both groups received dexamethasone 6mg for 10 days. Group allocation was blinded to the patient and research team. Primary outcomes were time to recovery, defined as the number of days until patients achieved WHO Ordinal Scale (OS) category ≤ 3, and the effect of spironolactone on aldosterone, D-dimer, angiotensin II and Von Willebrand Factor (VWF).</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>120 patients were recruited in Delhi from 01 February to 30 April 2021. 74 were randomly assigned to spironolactone and dexamethasone (SpiroDex), and 46 to dexamethasone alone (Dex). There was no significant difference in the time to recovery between SpiroDex and Dex groups (SpiroDex median 4.5 days, Dex median 5.5 days, p = 0.055). SpiroDex patients had lower aldosterone levels on day 7 and lower D-dimer levels on days 4 and 7 (day 7 D-dimer mean SpiroDex 1.15µg/mL, Dex 3.15 µg/mL, p = 0.0004). There was no increase in adverse events in patients receiving SpiroDex. <jats:italic>Post hoc</jats:italic> analysis demonstrated reduced clinical deterioration (pre specified as escalating to WHO OS category >4) in the SpiroDex group vs Dex group (5.4% vs 19.6%).</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Low dose oral spironolactone in addition to dexamethasone was safe and reduced D-Dimer and aldosterone. Although time to recovery was not significantly reduced, fewer patients progressed to severe disease. Phase 3 randomised controlled trials with spironolactone should be considered.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2022,
7,
2
]
]
},
"author": [
{
"affiliation": [],
"family": "Wadhwa",
"given": "Bharti",
"sequence": "first"
},
{
"affiliation": [],
"family": "Malhotra",
"given": "Vikas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kerai",
"given": "Sukhyanti",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Husain",
"given": "Farah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pandey",
"given": "Nalini Bala",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saxena",
"given": "Kirti N",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Vinay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Quinn",
"given": "Tom M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Feng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gaughan",
"given": "Erin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shankar-Hari",
"given": "Manu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mills",
"given": "Bethany",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Antonelli",
"given": "Jean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bruce",
"given": "Annya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Finlayson",
"given": "Keith",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moore",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dhaliwal",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Edwards",
"given": "Christopher",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
7,
4
]
],
"date-time": "2022-07-04T15:20:14Z",
"timestamp": 1656948014000
},
"deposited": {
"date-parts": [
[
2022,
7,
4
]
],
"date-time": "2022-07-04T15:20:18Z",
"timestamp": 1656948018000
},
"group-title": "Respiratory Medicine",
"indexed": {
"date-parts": [
[
2022,
7,
4
]
],
"date-time": "2022-07-04T15:42:21Z",
"timestamp": 1656949341013
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
7,
2
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.07.01.22277163",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
7,
2
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
7,
2
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1016/j.clim.2020.108448",
"doi-asserted-by": "publisher",
"key": "2022070408200497000_2022.07.01.22277163v1.1"
},
{
"DOI": "10.1186/s13054-021-03492-x",
"article-title": "Causes and timing of death in critically ill COVID-19 patients",
"doi-asserted-by": "crossref",
"first-page": "79",
"issue": "1",
"journal-title": "Critical Care",
"key": "2022070408200497000_2022.07.01.22277163v1.2",
"volume": "25",
"year": "2021"
},
{
"article-title": "Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19",
"first-page": "e00754",
"issue": "6",
"journal-title": "Antimicrobial Agents and Chemotherapy",
"key": "2022070408200497000_2022.07.01.22277163v1.3",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1126/science.abb2762",
"doi-asserted-by": "publisher",
"key": "2022070408200497000_2022.07.01.22277163v1.4"
},
{
"DOI": "10.1016/j.jaad.2020.04.032",
"doi-asserted-by": "publisher",
"key": "2022070408200497000_2022.07.01.22277163v1.5"
},
{
"DOI": "10.1038/s41467-021-24342-y",
"article-title": "The antiandrogen enzalutamide downregulates TMPRSS2 and reduces cellular entry of SARS-CoV-2 in human lung cells",
"doi-asserted-by": "crossref",
"first-page": "4068",
"issue": "1",
"journal-title": "Nature Communications",
"key": "2022070408200497000_2022.07.01.22277163v1.6",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.ejim.2020.04.037",
"doi-asserted-by": "publisher",
"key": "2022070408200497000_2022.07.01.22277163v1.7"
},
{
"article-title": "New Horizons: Does Mineralocorticoid Receptor Activation by Cortisol Cause ATP Release and COVID-19 Complications?",
"first-page": "622",
"issue": "3",
"journal-title": "The Journal of Clinical Endocrinology & Metabolism",
"key": "2022070408200497000_2022.07.01.22277163v1.8",
"volume": "106",
"year": "2020"
},
{
"DOI": "10.1016/j.imlet.2003.11.008",
"doi-asserted-by": "publisher",
"key": "2022070408200497000_2022.07.01.22277163v1.9"
},
{
"DOI": "10.1371/journal.pone.0081090",
"doi-asserted-by": "publisher",
"key": "2022070408200497000_2022.07.01.22277163v1.10"
},
{
"DOI": "10.3390/jcm9092943",
"doi-asserted-by": "crossref",
"key": "2022070408200497000_2022.07.01.22277163v1.11",
"unstructured": "Vicenzi M , Ruscica M , Iodice S , Rota I , Ratti A , Di Cosola R , et al. The Efficacy of the Mineralcorticoid Receptor Antagonist Canrenone in COVID-19 Patients. J Clin Med. 2020;9(9)."
},
{
"DOI": "10.3389/fendo.2021.747744",
"doi-asserted-by": "crossref",
"key": "2022070408200497000_2022.07.01.22277163v1.12",
"unstructured": "Edwards C , Klekot O , Halugan L , Korchev Y. Follow Your Nose: A Key Clue to Understanding and Treating COVID-19. Front Endocrinol (Lausanne). 2021;12(1528)."
},
{
"key": "2022070408200497000_2022.07.01.22277163v1.13",
"unstructured": "Physicians RCo . National Early Warning Score (NEWS) 2: Standardising the assessment of acute-illness severity in the NHS. Updated report of a working party. London: RCP: Royal College of Physicians; 2017."
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"doi-asserted-by": "publisher",
"key": "2022070408200497000_2022.07.01.22277163v1.14"
},
{
"DOI": "10.1016/j.diabres.2020.108293",
"doi-asserted-by": "publisher",
"key": "2022070408200497000_2022.07.01.22277163v1.15"
},
{
"DOI": "10.1111/jth.14768",
"doi-asserted-by": "publisher",
"key": "2022070408200497000_2022.07.01.22277163v1.16"
},
{
"DOI": "10.1016/SO140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "2022070408200497000_2022.07.01.22277163v1.17"
}
],
"reference-count": 17,
"references-count": 17,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2022.07.01.22277163"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Phase 2 randomised placebo-controlled trial of spironolactone and dexamethasone versus dexamethasone in COVID-19 hospitalised patients in Delhi",
"type": "posted-content"
}