Voxvoganan (LTX-109, Lytixar) is a synthetic cationic amphipathic antimicrobial peptidomimetic (small peptide-mimetic) designed to mimic host-defense peptides.
May 1 2022 |
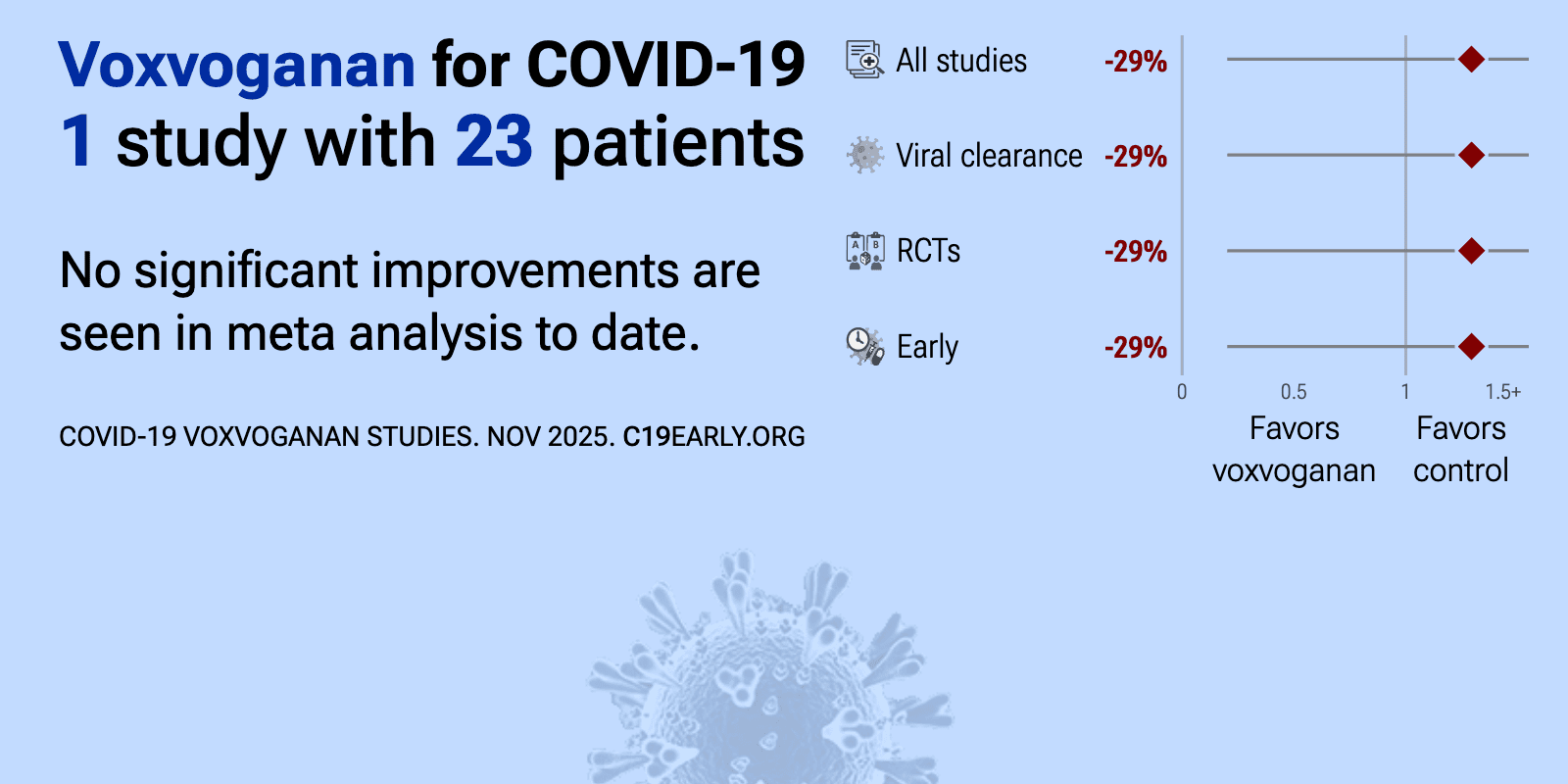
et al., NCT04854928 | A Double-blind, Placebo-controlled, Interventional Parallel Group Study to Evaluate the Antiviral Effect of a Single Nasal Application of LTX-109 3% Gel, in Comparison to Placebo Gel, in Subjects With COVID-19 Infection. |
| 29% worse viral clearance (p=0.8). RCT 33 patients up to 6 days from symptom onset, showing no significant difference in short-term viral load change with LTX-109. | ||