Early experimental COVID-19 therapies: associations with length of hospital stay, mortality and related costs
et al., Swiss Medical Weekly, doi:10.4414/smw.2020.20446 , Dec 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
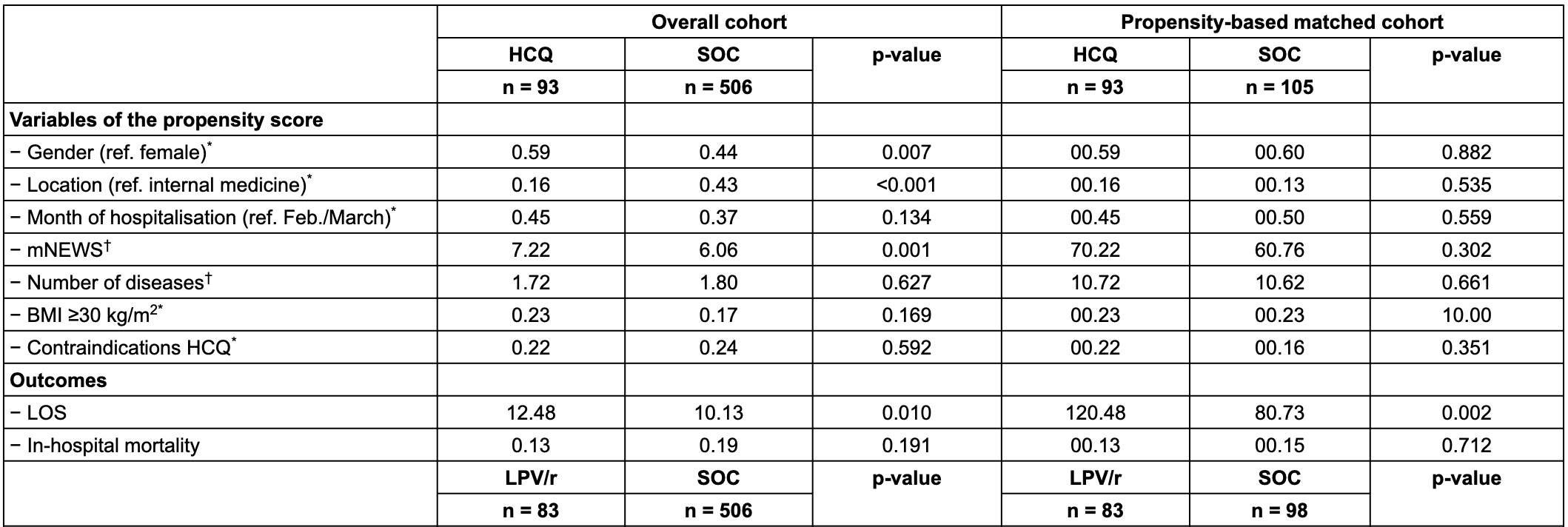
Retrospective 840 hospitalized patients in Switzerland showing non-statistically significant lower mortality with HCQ but significantly longer hospitalization times. Confounding by indication is likely. PSM fails to adjust for severity with a 16% higher mNEWS score for HCQ vs. SOC in the matched cohort.
Time varying confounding is likely. HCQ became controversial and was suspended towards the end of the period studied, therefore HCQ use was likely more frequent toward the beginning of the study period, a time when overall treatment protocols were significantly worse.
Authors note: "overall, there was an indication bias, with the reason of prescription being associated with the outcome of interest. Indeed, patients with more severe COVID-19 were more likely to receive experimental therapies."
This study is excluded in the after exclusion results of meta-analysis:
substantial confounding by time likely due to declining usage over the early stages of the pandemic when overall treatment protocols improved dramatically; substantial unadjusted confounding by indication likely.
|
risk of death, 15.3% lower, RR 0.85, p = 0.71, treatment 12 of 93 (12.9%), control 16 of 105 (15.2%), NNT 43, HCQ vs. SOC, PSM.
|
|
hospitalization time, 49.0% higher, relative time 1.49, p = 0.002, treatment 93, control 105, HCQ vs. SOC, PSM.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Vernaz et al., 31 Dec 2020, retrospective, propensity score matching, Switzerland, peer-reviewed, 15 authors.
DOI record:
{
"DOI": "10.4414/smw.2020.20446",
"ISSN": [
"1424-3997"
],
"URL": "http://dx.doi.org/10.4414/smw.2020.20446",
"abstract": "<jats:p>AIMS OF THE STUDY\n Hydroxychloroquine and lopinavir/ritonavir have been used as experimental therapies to treat COVID-19 during the first wave of the pandemic. Randomised controlled trials have recently shown that there are no meaningful benefits of these two therapies in hospitalised patients. Uncertainty remains regarding the potential harmful impact of these therapies as very early treatments and their burden to the health care system. The present study investigated the length of hospital stay (LOS), mortality, and costs of hydroxychloroquine, lopinavir/ritonavir or their combination in comparison with standard of care among patients hospitalised for coronavirus disease 2019 (COVID-19).\n \n \n METHODS\n This retrospective observational cohort study took place in the Geneva University Hospitals, Geneva, Switzerland (n = 840) between 26 February and 31 May 2020. Demographics, treatment regimens, comorbidities, the modified National Early Warning Score (mNEWS) on admission, and contraindications to COVID-19 treatment options were assessed. Outcomes included LOS, in-hospital mortality, and drug and LOS costs.\n \n \n RESULTS\n After successful propensity score matching, patients treated with (1) hydroxychloroquine, (2) lopinavir/ritonavir or (3) their combination had on average 3.75 additional hospitalisation days (95% confidence interval [CI] 1.37–6.12, p = 0.002), 1.23 additional hospitalisation days (95% CI −1.24 – 3.51, p = 0.319), and 4.19 additional hospitalisation days (95% CI 1.52–5.31, p <0.001), respectively, compared with patients treated with the standard of care. Neither experimental therapy was significantly associated with mortality. These additional hospital days amounted to 1010.77 additional days for hydroxychloroquine and hydroxychloroquine combined with lopinavir/ritonavir, resulting in an additional cost of US$ 2,492,214 (95%CI US$ 916,839–3,450,619).\n \n \n CONCLUSIONS\n Prescribing experimental therapies for COVID-19 was not associated with a reduced LOS and might have increased the pressure put on healthcare systems.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Vernaz",
"given": "Nathalie",
"sequence": "first"
},
{
"affiliation": [],
"family": "Agoritsas",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Calmy",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gayet-Ageron",
"given": "Angèle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gold",
"given": "Gabriel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perrier",
"given": "Arnaud",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Picard",
"given": "Fabienne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prendki",
"given": "Virginie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reny",
"given": "Jean-Luc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Samer",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stirnemann",
"given": "Jérôme",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vetter",
"given": "Pauline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zanella",
"given": "Marie-Céline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zekry",
"given": "Dina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baggio",
"given": "Stéphanie",
"sequence": "additional"
}
],
"container-title": "Swiss Medical Weekly",
"container-title-short": "Swiss Med Wkly",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
12,
31
]
],
"date-time": "2020-12-31T16:40:38Z",
"timestamp": 1609432838000
},
"deposited": {
"date-parts": [
[
2023,
1,
26
]
],
"date-time": "2023-01-26T22:38:25Z",
"timestamp": 1674772705000
},
"indexed": {
"date-parts": [
[
2024,
2,
9
]
],
"date-time": "2024-02-09T07:31:20Z",
"timestamp": 1707463880067
},
"is-referenced-by-count": 20,
"issue": "5153",
"issued": {
"date-parts": [
[
2020,
12,
31
]
]
},
"journal-issue": {
"issue": "5153",
"published-online": {
"date-parts": [
[
2020,
12,
14
]
]
}
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-sa/4.0",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
12,
31
]
],
"date-time": "2020-12-31T00:00:00Z",
"timestamp": 1609372800000
}
}
],
"link": [
{
"URL": "https://smw.ch/index.php/smw/article/download/2919/4793",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://smw.ch/index.php/smw/article/download/2919/4794",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://smw.ch/index.php/smw/article/download/2919/4794",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "36756",
"original-title": [],
"page": "w20446",
"prefix": "10.57187",
"published": {
"date-parts": [
[
2020,
12,
31
]
]
},
"published-online": {
"date-parts": [
[
2020,
12,
31
]
]
},
"publisher": "SMW Supporting Association",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://smw.ch/index.php/smw/article/view/2919"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Early experimental COVID-19 therapies: associations with length of hospital stay, mortality and related costs",
"type": "journal-article",
"volume": "150"
}
