Heat-killed Lactobacillus acidophilus suppresses SARS-CoV-2 infection in the human intestinal epithelial cell line Caco-2
et al., Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2025.1556344, Jul 2025
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
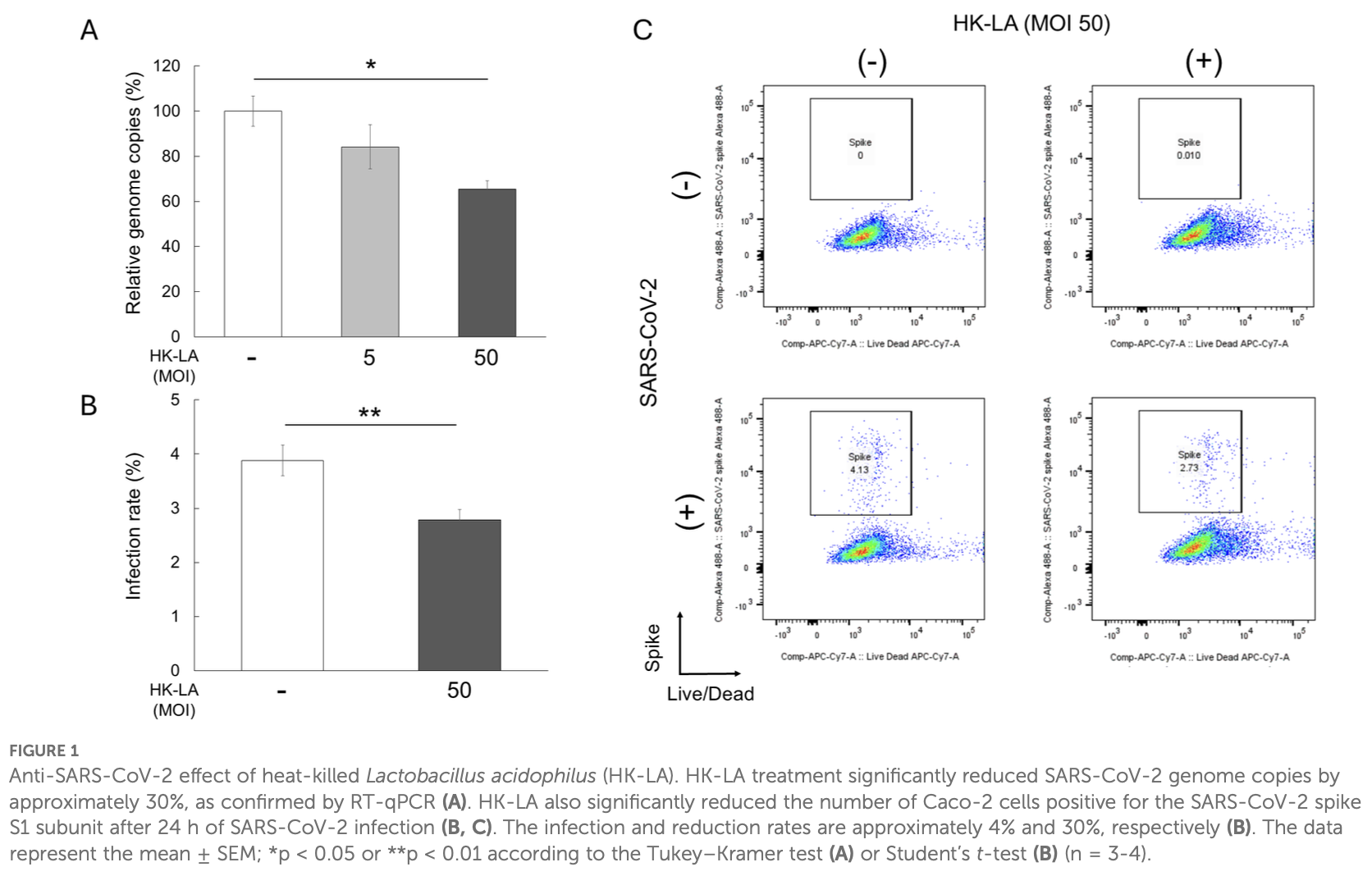
In vitro study showing that heat-killed Lactobacillus acidophilus (HK-LA) reduces SARS-CoV-2 infection by approximately 30% in Caco-2 human intestinal epithelial cells. Authors found that HK-LA treatment significantly reduced viral genome copies and the number of spike-positive infected cells compared to controls. The protective effect was associated with increased secretion of interferon lambda-2 (IFN-λ2), while ACE2 and TMPRSS2 receptor expression remained unchanged.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
Takada et al., 31 Jul 2025, Japan, peer-reviewed, 6 authors.
Contact: takada.kazuhide@nihon-u.ac.jp, aizawa.shihoko@nihon-u.ac.jp.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Heat-killed Lactobacillus acidophilus suppresses SARS-CoV-2 infection in the human intestinal epithelial cell line Caco-2
Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2025.1556344
Background: The gastrointestinal (GI) tract is suspected to be a possible source for the systemic spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as well as a reservoir of long coronavirus disease (COVID). Thus, the mucosal epithelial tissue of the colon is a potential target for probiotics to help control SARS-CoV-2 infection. Recently, the effect of live probiotics on COVID-19 has been evaluated. However, live probiotics have certain risks, including the transmission of antibiotic-resistant genes, disturbance of gut colonization in infants, and systemic infections induced by translocation. Therefore, there is growing interest in nonviable microorganisms, particularly heat-killed probiotic bacteria, to mitigate these risks. Methods: This study evaluated the antiviral properties of heat-killed Lactobacillus acidophilus (HK-LA) in the Caco-2 cell line. Caco-2 cells were infected by SARS-CoV-2 with or without 24-hour pretreatment of HK-LA and the presence of HK-LA during infection. Results: RT-qPCR analysis showed that HK-LA treatment significantly reduced SARS-CoV-2 genome copies by approximately 30%. Similarly, flow cytometry revealed a roughly 30% decrease in SARS-CoV-2 spike-positive Caco-2 cells following HK-LA treatment. Additionally, ELISA demonstrated a significant increase in IFN-l2 secretion induced by HK-LA. Discussion: HK-LA reduces viral infection in Caco-2 cells with an increase in IFN-l2 secretion. Therefore, heat-killed lactobacilli could potentially reduce SARS-CoV-2 infection in the GI tract, suggesting a possible clinical application.
Ethics statement Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
Generative AI statement The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025. 1556344/full#supplementary-material
References
Abramov, Kosarev, Priputnevich, Machulin, Abashina et al., S-layer protein 2 of vaginal Lactobacillus crispatus 2029 enhances growth, differentiation, VEGF production and barrier functions in intestinal epithelial cell line Caco-2, Int. J. Biol. Macromol, doi:10.1016/j.ijbiomac.2021.08.150
Almeida Viana, Do Reis Santos, Pereira, De Carvalho Alves, Tianeze De Castro et al., Benefits of probiotic use on COVID-19: A systematic review and meta-analysis, Crit. Rev. Food Sci. Nutr, doi:10.1080/10408398.2022.2128713
Felgenhauer, Schoen, Gad, Hartmann, Schaubmar et al., Inhibition of SARS-CoV-2 by type I and type III interferons, J. Biol. Chem, doi:10.1074/jbc.AC120.013788
Hayashida, Takada, Melnikov, Komine-Aizawa, Tsuji et al., How were Lactobacillus species selected as single dominant species in the human vaginal microbiota? Coevolution of humans and Lactobacillus, Med. Hypotheses, doi:10.1016/j.mehy.2022.110858
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-coV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Mahooti, Miri, Abdolalipour, Ghaemi, The immunomodulatory effects of probiotics on respiratory viral infections: A hint for COVID-19 treatment?, Microbial, doi:10.1016/j.micpath.2020.104452
Neurath, Überla, Ng, Gut as viral reservoir: lessons from gut viromes, HIV and COVID-19, Gut, doi:10.1136/gutjnl-2021-324622
Nguyen, Chong, Hor, Lew, Rather et al., Role of probiotics in the management of COVID-19: A computational perspective, Nutrients, doi:10.3390/nu14020274
Pique, Berlanga, Miñana-Galbis, Health benefits of heat-killed (tyndallized) probiotics: An overview, Int. J. Mol. Sci, doi:10.3390/ijms20102534
Saito, Kakizaki, Okuno, Maekawa, Tsuji, Lactococcus lactis subsp. Cremoris C60 restores T Cell population in small intestinal lamina propria in aged interleukin-18 deficient mice, Nutrients, doi:10.3390/nu12113287
Salaris, Scarpa, Elli, Bertolini, Guglielmetti et al., Lacticaseibacillus paracasei DG enhances the lactoferrin anti-SARS-CoV-2 response in Caco-2 cells, Gut. Microbes
Stanifer, Kee, Cortese, Zumaran, Triana et al., Critical role of type III interferon in controlling SARS-CoV-2 infection in human intestinal epithelial cells, Cell Rep, doi:10.1016/j.celrep.2020.107863
Suryawanshi, Biswas, Herd Immunity to fight against COVID-19: A narrative review, Cureus, doi:10.7759/cureus.33575
Taufer, Da Silva, Rampelotto, The influence of probiotic Lactobacilli on COVID-19 and the microbiota, Nutrients, doi:10.3390/nu16091350
Vanderheiden, Ralfs, Chirkova, Upadhyay, Zimmerman et al., Type I and type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures, J. Virol, doi:10.1128/JVI.00985-20
Wise, Covid-19: WHO declares end of global health emergency, BMJ (Clinical. Res. ed, doi:10.1136/bmj.p1041
Wu, Xiang, Jing, Wang, Novakovic et al., Damage to endothelial barriers and its contribution to long COVID, Angiogenesis, doi:10.1007/s10456-023-09878-5
DOI record:
{
"DOI": "10.3389/fcimb.2025.1556344",
"ISSN": [
"2235-2988"
],
"URL": "http://dx.doi.org/10.3389/fcimb.2025.1556344",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>The gastrointestinal (GI) tract is suspected to be a possible source for the systemic spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as well as a reservoir of long coronavirus disease (COVID). Thus, the mucosal epithelial tissue of the colon is a potential target for probiotics to help control SARS-CoV-2 infection. Recently, the effect of live probiotics on COVID-19 has been evaluated. However, live probiotics have certain risks, including the transmission of antibiotic-resistant genes, disturbance of gut colonization in infants, and systemic infections induced by translocation. Therefore, there is growing interest in nonviable microorganisms, particularly heat-killed probiotic bacteria, to mitigate these risks.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>This study evaluated the antiviral properties of heat-killed <jats:italic>Lactobacillus acidophilus</jats:italic> (HK-LA) in the Caco-2 cell line. Caco-2 cells were infected by SARS-CoV-2 with or without 24-hour pretreatment of HK-LA and the presence of HK-LA during infection.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>RT-qPCR analysis showed that HK-LA treatment significantly reduced SARS-CoV-2 genome copies by approximately 30%. Similarly, flow cytometry revealed a roughly 30% decrease in SARS-CoV-2 spike-positive Caco-2 cells following HK-LA treatment. Additionally, ELISA demonstrated a significant increase in IFN-λ2 secretion induced by HK-LA.</jats:p></jats:sec><jats:sec><jats:title>Discussion</jats:title><jats:p>HK-LA reduces viral infection in Caco-2 cells with an increase in IFN-λ2 secretion. Therefore, heat-killed lactobacilli could potentially reduce SARS-CoV-2 infection in the GI tract, suggesting a possible clinical application.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fcimb.2025.1556344"
],
"article-number": "1556344",
"author": [
{
"affiliation": [],
"family": "Takada",
"given": "Kazuhide",
"sequence": "first"
},
{
"affiliation": [],
"family": "Trinh",
"given": "Quang Duy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Takeda",
"given": "Yoshinori",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsuji",
"given": "Noriko M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hayakawa",
"given": "Satoshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Komine-Aizawa",
"given": "Shihoko",
"sequence": "additional"
}
],
"container-title": "Frontiers in Cellular and Infection Microbiology",
"container-title-short": "Front. Cell. Infect. Microbiol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2025,
7,
31
]
],
"date-time": "2025-07-31T05:38:49Z",
"timestamp": 1753940329000
},
"deposited": {
"date-parts": [
[
2025,
7,
31
]
],
"date-time": "2025-07-31T05:38:50Z",
"timestamp": 1753940330000
},
"indexed": {
"date-parts": [
[
2025,
8,
2
]
],
"date-time": "2025-08-02T19:38:45Z",
"timestamp": 1754163525719,
"version": "3.41.2"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
7,
31
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
7,
31
]
],
"date-time": "2025-07-31T00:00:00Z",
"timestamp": 1753920000000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fcimb.2025.1556344/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2025,
7,
31
]
]
},
"published-online": {
"date-parts": [
[
2025,
7,
31
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1016/j.ijbiomac.2021.08.150",
"article-title": "S-layer protein 2 of vaginal Lactobacillus crispatus 2029 enhances growth, differentiation, VEGF production and barrier functions in intestinal epithelial cell line Caco-2",
"author": "Abramov",
"doi-asserted-by": "publisher",
"first-page": "410",
"journal-title": "Int. J. Biol. Macromol.",
"key": "B1",
"volume": "189",
"year": "2021"
},
{
"DOI": "10.1074/jbc.AC120.013788",
"article-title": "Inhibition of SARS-CoV-2 by type I and type III interferons",
"author": "Felgenhauer",
"doi-asserted-by": "publisher",
"first-page": "13958",
"journal-title": "J. Biol. Chem.",
"key": "B2",
"volume": "295",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2022.110858",
"article-title": "How were Lactobacillus species selected as single dominant species in the human vaginal microbiota? Coevolution of humans and Lactobacillus",
"author": "Hayashida",
"doi-asserted-by": "publisher",
"first-page": "110858",
"journal-title": "Med. Hypotheses.",
"key": "B3",
"volume": "163",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-coV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "271",
"journal-title": "Cell",
"key": "B4",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.micpath.2020.104452",
"article-title": "The immunomodulatory effects of probiotics on respiratory viral infections: A hint for COVID-19 treatment",
"author": "Mahooti",
"doi-asserted-by": "publisher",
"first-page": "104452",
"journal-title": "Microbial. Pathogene.",
"key": "B5",
"volume": "148",
"year": "2020"
},
{
"DOI": "10.1080/10408398.2022.2128713",
"article-title": "Benefits of probiotic use on COVID-19: A systematic review and meta-analysis",
"author": "Neris Almeida Viana",
"doi-asserted-by": "publisher",
"first-page": "2986",
"journal-title": "Crit. Rev. Food Sci. Nutr.",
"key": "B6",
"volume": "64",
"year": "2024"
},
{
"DOI": "10.1136/gutjnl-2021-324622",
"article-title": "Gut as viral reservoir: lessons from gut viromes, HIV and COVID-19",
"author": "Neurath",
"doi-asserted-by": "publisher",
"first-page": "1605",
"journal-title": "Gut",
"key": "B7",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.3390/nu14020274",
"article-title": "Role of probiotics in the management of COVID-19: A computational perspective",
"author": "Nguyen",
"doi-asserted-by": "publisher",
"first-page": "274",
"journal-title": "Nutrients",
"key": "B8",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3390/ijms20102534",
"article-title": "Health benefits of heat-killed (tyndallized) probiotics: An overview",
"author": "Piqué",
"doi-asserted-by": "publisher",
"first-page": "2534",
"journal-title": "Int. J. Mol. Sci.",
"key": "B9",
"volume": "20",
"year": "2019"
},
{
"DOI": "10.3390/nu12113287",
"article-title": "Lactococcus lactis subsp. Cremoris C60 restores T Cell population in small intestinal lamina propria in aged interleukin-18 deficient mice",
"author": "Saito",
"doi-asserted-by": "publisher",
"first-page": "3287",
"journal-title": "Nutrients",
"key": "B10",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1080/19490976.2021.1961970",
"article-title": "Lacticaseibacillus paracasei DG enhances the lactoferrin anti-SARS-CoV-2 response in Caco-2 cells",
"author": "Salaris",
"doi-asserted-by": "crossref",
"first-page": "1961970",
"journal-title": "Gut. Microbes",
"key": "B11",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.celrep.2020.107863",
"article-title": "Critical role of type III interferon in controlling SARS-CoV-2 infection in human intestinal epithelial cells",
"author": "Stanifer",
"doi-asserted-by": "publisher",
"first-page": "107863",
"journal-title": "Cell Rep.",
"key": "B12",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.7759/cureus.33575",
"article-title": "Herd Immunity to fight against COVID-19: A narrative review",
"author": "Suryawanshi",
"doi-asserted-by": "publisher",
"journal-title": "Cureus",
"key": "B13",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.3390/nu16091350",
"article-title": "The influence of probiotic Lactobacilli on COVID-19 and the microbiota",
"author": "Taufer",
"doi-asserted-by": "publisher",
"first-page": "1350",
"journal-title": "Nutrients",
"key": "B14",
"volume": "16",
"year": "2024"
},
{
"DOI": "10.1128/JVI.00985-20",
"article-title": "Type I and type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures",
"author": "Vanderheiden",
"doi-asserted-by": "publisher",
"journal-title": "J. Virol.",
"key": "B15",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.1136/bmj.p1041",
"article-title": "Covid-19: WHO declares end of global health emergency",
"author": "Wise",
"doi-asserted-by": "publisher",
"first-page": "1041",
"journal-title": "BMJ (Clinical. Res. ed).",
"key": "B16",
"volume": "381",
"year": "2023"
},
{
"DOI": "10.1007/s10456-023-09878-5",
"article-title": "Damage to endothelial barriers and its contribution to long COVID",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "5",
"journal-title": "Angiogenesis",
"key": "B17",
"volume": "27",
"year": "2024"
}
],
"reference-count": 17,
"references-count": 17,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fcimb.2025.1556344/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Heat-killed Lactobacillus acidophilus suppresses SARS-CoV-2 infection in the human intestinal epithelial cell line Caco-2",
"type": "journal-article",
"update-policy": "https://doi.org/10.3389/crossmark-policy",
"volume": "15"
}
